Prerequisites for effective spread of HIV-1 in humans
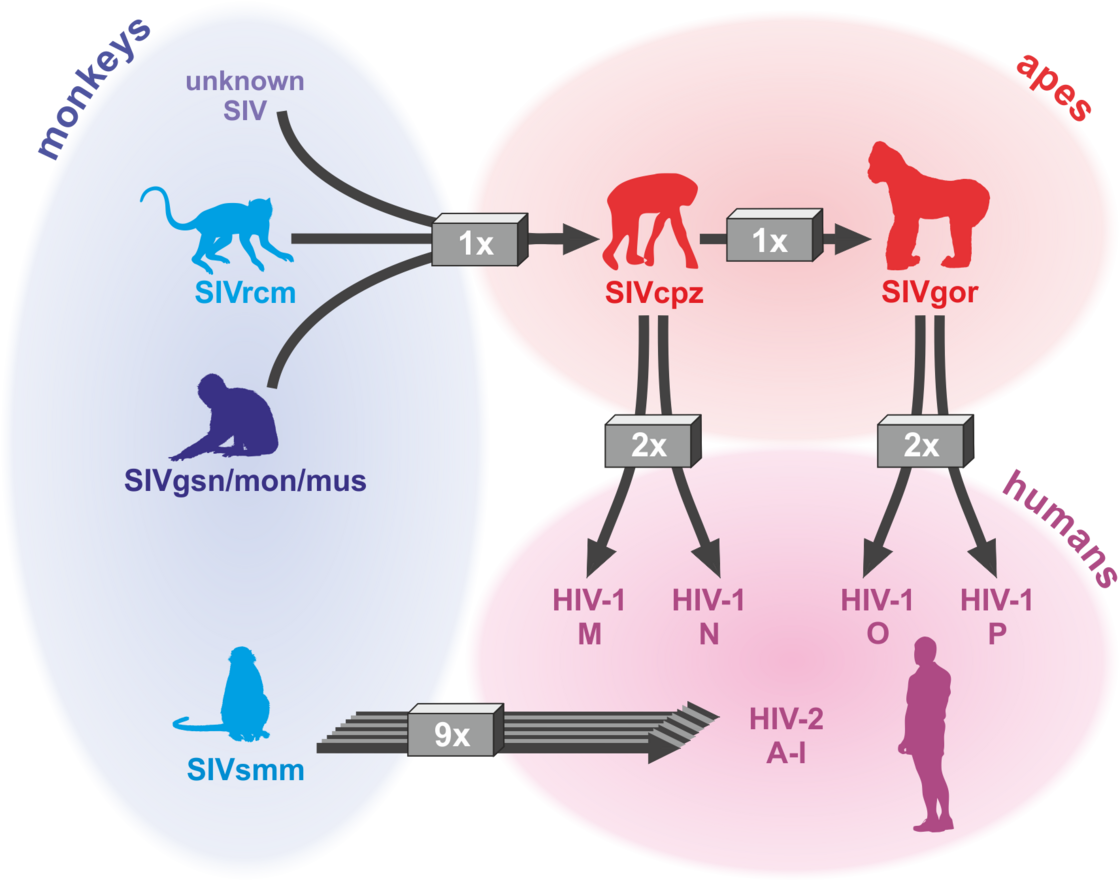
The AIDS pandemic is the consequence of zoonotic transmissions of simian immunodeficiency viruses (SIVs) infecting >40 monkey and ape species in sub-Saharan Africa. Four groups of HIV-1 (M, N, O and P) and nine groups of HIV-2 (A-I) all resulting from independent transmission events have been described. However, only HIV-1 group M that originated from a single transmission event of SIVcpz infecting chimpanzees is responsible for the AIDS pandemic. Numerous adaptations were required for efficient spread of HIV-1 in the human population (reviewed in Sauter & Kirchhoff, Cell Host & Microbe 2019). For example, we found that only HIV-1 M strains evolved a fully functional viral protein U (Vpu) that counteracts the antiviral factor “tetherin” that prevents the release of viral particles (Sauter et al., Cell Host & Microbe, 2009). In contrast, Vpus from rare group N and P strains gained little activity against this antiviral factor (Sauter et al., Retrovirology 2011; PLOS Pathogens 2012) and epidemic HIV-1 group O strains evolved an alternative but less effective mechanism (Kluge et al., Cell Host & Microbe 2014). Tetherin represents an effective barrier against primate lentiviral zoonoses because the human variant of tetherin lacks a small region, which is used by most SIV Nef proteins of the monkey immunodeficiency virus to neutralize this antiviral factor. In cooperative studies, we determined the structural basis for tetherin antagonism by HIV-1 O and SIV Nef proteins (Morris et al., Cell 2018; Buffalo et al., Cell Host & Microbe 2019) and showed that tetherin antagonism is most likely critical for transmission fitness of HIV-1 (Kmiec et al., mBio 2016; Yamada et al., Cell Host & Microbe 2018). We also identified novel restriction factors targeting the HIV-1 RNA, virus transcription or Env function that might play key roles in viral replication and latency (Krapp et al., Cell Host & Microbe 2016; Braun et al., Cell Reports 2019; Hotter et al., Cell Host & Microbe 2019; Yamasoba et al., Nature Microb. 2019).
HIV/AIDS – origin: at least 13 zoonotic transmission of SIVs (Sauter & Kirchhoff, Cell Host & Microbe 2019)
Determinants of HIV-1 pathogenicity
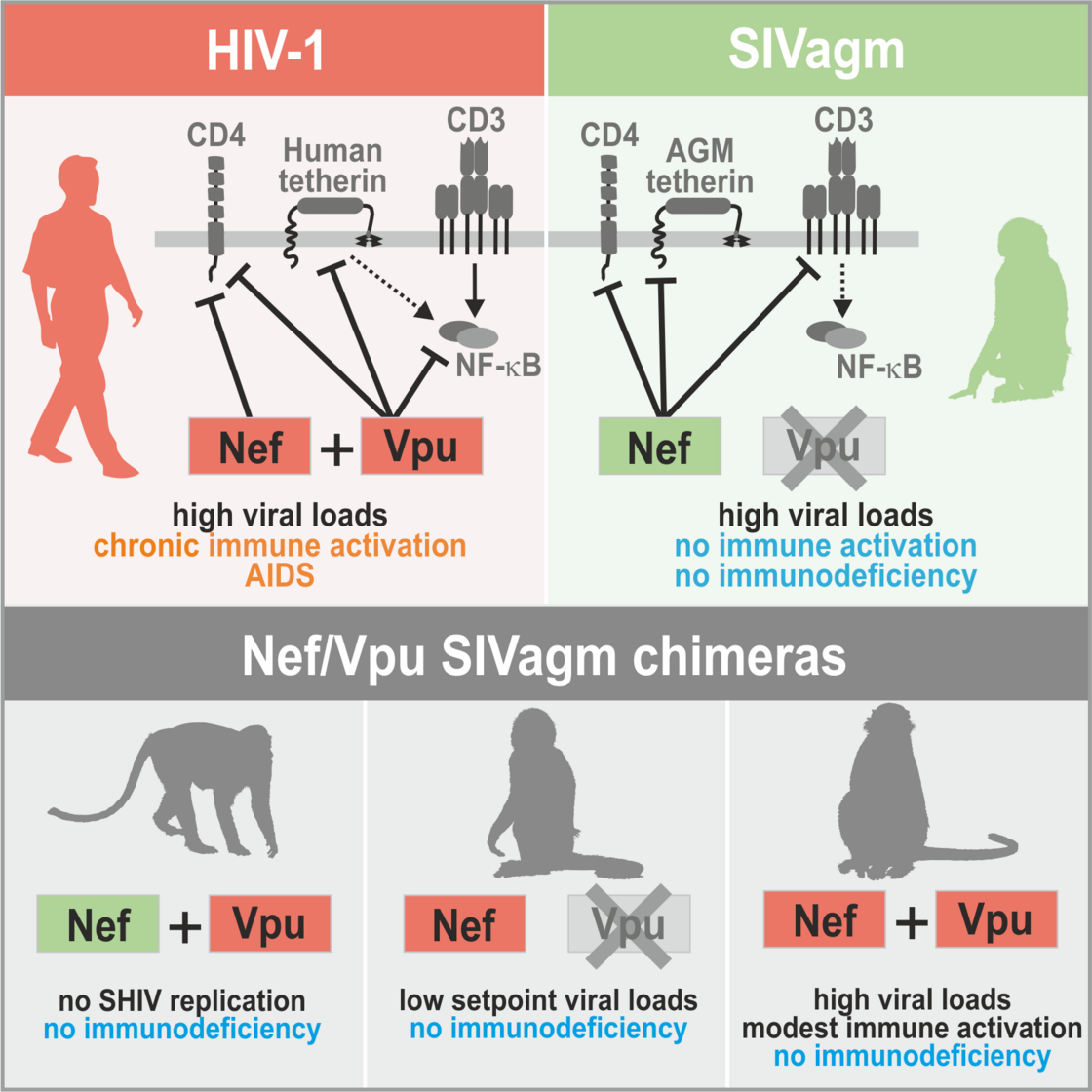
In contrast to HIV-1, SIVs do usually not cause disease in their well-adapted non-human hosts. We are interested in the determinants of the high virulence of HIV-1. In early studies, we showed that the multifunctional viral factor Nef plays a key role in HIV-1 replication and pathogenesis. High levels of chronic inflammation drive progression to AIDS in humans and are absent in non-pathogenic SIV infection. We found that HIV-1 Nef proteins boost T cell activation, while most SIVs block it and suppress inflammatory responses (Schindler et al., Cell 2006). Thus, loss of a protective Nef function helps to explain the high virulence of HIV-1 (Kirchhoff, Nat. Rev. Microbiol. 2009). Recently, we generated “HIV-1-like” SIV constructs to show that characteristic features of HIV-1 contribute to the high levels of chronic inflammation that drive progression to AIDS in humans (Joas et al., Nature Com. 2018). However, they do not overcome protective mechanisms evolved by well-adapted natural hosts of SIV, such as blunted responses to TLR-4 ligands (Palesch et al., Nature 2018).
Role of HIV-1-characteristic features for inflammation and pathogenesis (Joas et al., Nature Com. 2018)
Discovery and optimization of endogenous human factors modulating viral infections
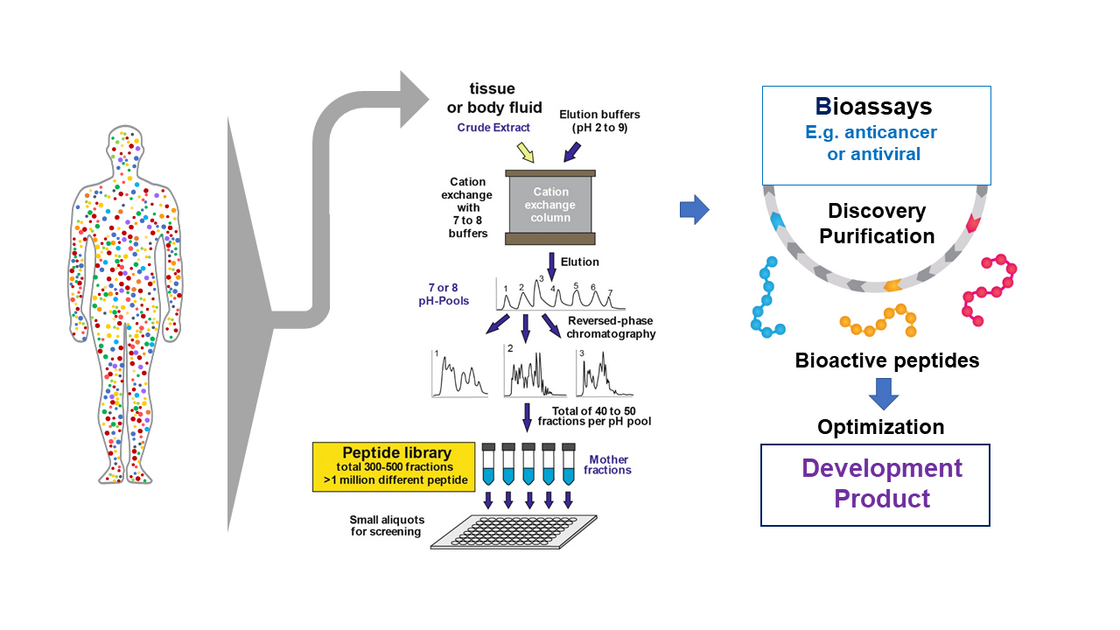
Another major interest of Kirchhoff`s group is exploitation of the human peptidome for novel antiviral and anticancer agents. The peptidome (i.e. the entirety of peptides in the body) comprises millions of agents including key immune regulators and effectors (reviewed in Münch et al., Nat Rev Microbiol. 2014; Bosso et al., Bioorg Med Chem. 2018). Only a small fraction has been characterized and the peptidome represents an almost unlimited resource for the discovery of novel biomolecules. In cooperation with the groups of Prof. Münch and Dr. Ständker, his group screened complex peptide-protein libraries from natural sources, such as blood and semen, to discover several compounds affecting HIV infection. One factor blocked HIV-1 entry by preventing anchoring of HIV-1 particles to its target cells (Münch et al., Cell 2017a). An optimized derivative was safe and effective in phase I/II clinical study (Forssmann et al., Science Transl Med. 2010). His lab was also involved in the discovery that semen contains fibril-forming peptides that boost HIV-1 infection (Münch et al., Cell 2017a; Usmani et al., Nature Com. 2014) and provided a basis for the development of nanofibers allowing to improve retroviral gene transfer (Yolamanova et al., Nat. Nanotechnol. 2013). Interestingly, the nanofibers in semen allow macrophages to remove damaged sperm, consequently improving sperm quality (Roan et al., eLife 2017). In cooperative studies, his group identified a small fragment of the highly abundant albumin protein as specific inhibitor of CXCR4 (Zirafi et al., Cell Reports 2015). This chemokine receptor is not only a major entry cofactor of HIV-1 but also involved in many cancers and inflammatory diseases. Recent data show that this endogenous peptide suppresses cancer cell metastasis and inflammatory responses. Optimized derivatives thereof are currently evaluated in preclinical trial for the treatment of several cancers and inflammatory disorders.
Strategy: Discovery and Optimization of novel Bioactive Peptides (Münch et al., Nat Rev Microbiol. 2014).