Head

Prof. Mike-Andrew Westhoff
Ulm University Medical Center
Department of Pediatrics and Adolescent Medicine
Eythstr. 24
89075 Ulm, Germany
phone: +49-731-500-57495
e-mail: mike-andrew.westhoff@uniklinik-ulm.de
Prof. Dr. Klaus-Michael Debatin
Founder & Mentor
Mike-Andrew Westhoff leads this group’s research on brain tumours, with a particular emphasis on Glioblastoma in adults and Medulloblastoma in children. Building on long-standing expertise in molecular oncology and translational collaboration, his team integrates tumour biology with clinically relevant model systems and patient-derived resources to identify vulnerabilities that can be leveraged therapeutically.
A central theme is how programmed cell death pathways—especially apoptosis—shape treatment response. The group developed from the groundbreaking work on apoptosis that Prof. Klaus-Michael Debatin initiated at the department after he became director in the mid-1990s, and it continues this legacy by investigating how cell-death and survival signalling determine sensitivity or resistance to radio- and chemotherapy as well as targeted approaches.
Glioblastoma remains a major focus because it is among the most lethal tumours encountered in the clinic. It is the most frequent primary malignant brain tumour in adults. Despite maximal safe surgical resection followed by radiotherapy and chemotherapy, recurrence is common and overall survival is typically limited.
Medulloblastoma is a prototypical malignant paediatric brain tumour arising in the cerebellum and represents one of the most common solid tumours in children. Current multimodal therapy can be effective, but it often requires intensive treatment and survivors may face significant long-term sequelae; relapsed disease remains particularly challenging, underscoring the need for more precise and less toxic strategies.
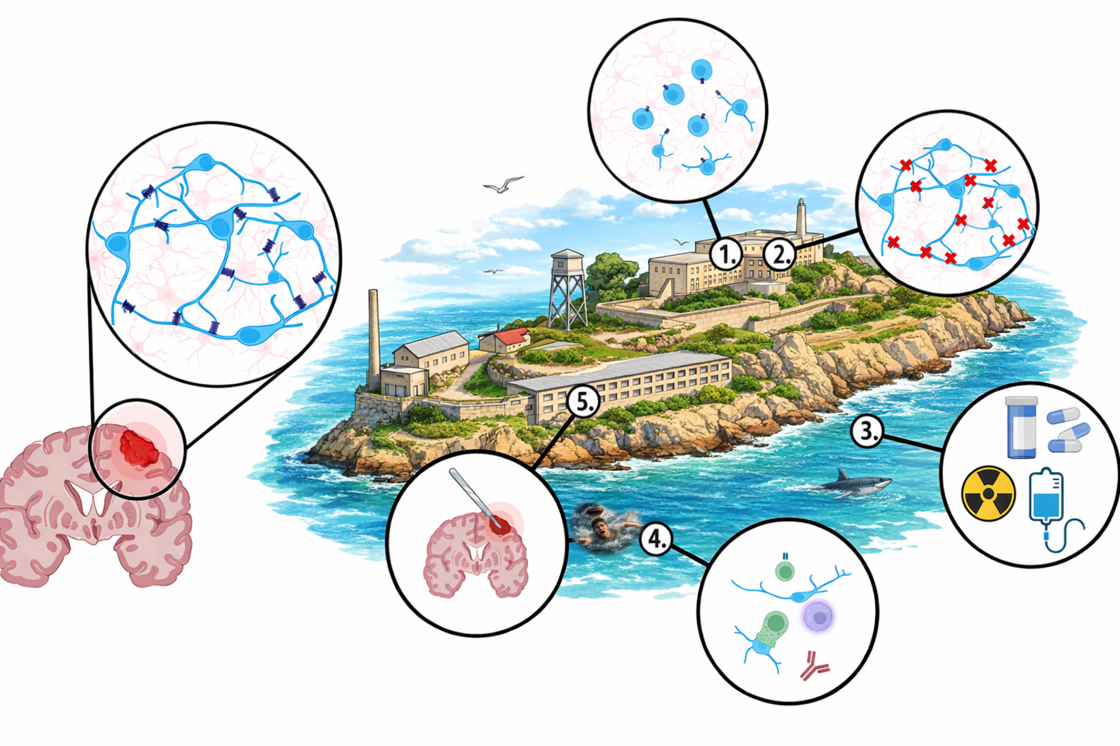
Figure Legend:
Glioblastoma (GBM) are highly invasive malignancies that in recent years have been shown to form a parasitic neuronal network within the healthy brain structure.
The Alcatraz Strategy is inspired by the (infamous) prison that laid concentric rings of isolation around the prisoners (one person per cell/cell in a prison on an island ice-cold, possibly shark-infested water around the island).
We do not want to completely isolate the cancer cell and take it out of its environment (1.) as this might make GBM cells more aggressive, but we aim to control the communication with the surrounding environment (2.), while controlling the effects standard therapy (3. And 5.) might have on cell motility. More recently, we added another potential component to the Alcatraz Strategy: Together with our partners from the Lyszkiewicz Group we are looking at the immune landscape of tumours to reactivate the sharks of the immune system to kill cancer cells (4.)
Despite its aggressiveness, glioblastoma often lacks a single dominant, easily druggable oncogenic driver; instead, many tumours converge on aberrant activation of the PI3K/Akt/mTOR signalling network, which is active in almost 90% of cases.
However, glioblastoma is not simply “PI3K-addicted”: pharmacological inhibition is frequently cytostatic and—if it reduces proliferation at the wrong time—can counteract chemotherapy, meaning that efficacy depends strongly on cell state and treatment schedule.
In patient-derived glioblastoma models, we found that PI3K signalling can have distinct, cell-specific functions within the same tumour—contributing to the enhanced therapy resistance of stem-like cells, while primarily regulating motility/invasion of more differentiated populations—highlighting why pathway inhibition must be integrated thoughtfully into rational, timed combination therapies, rather than applied as uniform maximal blockade.
Medulloblastoma (MB) is the most common malignant solid brain tumour in children. It typically arises in the cerebellum and is treated with an intensive multimodal regimen. While cure rates have improved, therapy can cause substantial long-term sequelae, and relapse remains difficult to treat. Molecularly, MB is an umbrella diagnosis comprising distinct subgroups (classically WNT, SHH, Group 3, Group 4) with different biology and prognosis.
A key translational concept we pursue in MB is to break intrinsic resistance to therapy-induced cell death so that effective treatment might be achievable with less toxic intensity, drawing from our decades-long experience in the field of apoptosis research.
In parallel, our broader brain-tumour program emphasizes that therapeutic success is also shaped by tumour–microenvironment interactions. Work on extracellular matrix cues such as reelin illustrates how modulating cell–matrix behaviour can alter malignant phenotypes (e.g., adhesion and motility) and provides a conceptual framework for adding microenvironment-focused interventions to cell-death–based strategies in brain tumours.
Our therapeutic work is guided by the idea that malignant brain tumours evolve under treatment pressure—and that therapy can be designed not only to “hit harder”, but to shape how tumour populations move through an adaptive (fitness) landscape. Instead of only creating a single deep “valley” by maximal cell kill, the goal is to steer evolutionary trajectories: anticipating which resistant peaks become accessible under a given intervention and then using sequence, timing, and combinations to constrain escape routes (“fitness landscape steering”).
One concrete translation of this evolutionary thinking is the RIST strategy—a metronomic combination regimen integrating targeted pathway inhibition with cytotoxic therapy (Rapamycin, Sunitinib, Irinotecan, Temozolomide). In glioblastoma models, this combination increased apoptosis and reduced proliferation compared with single agents, and in compassionate-use settings it was able to stabilize tumour burden for prolonged periods in selected, heavily pretreated patients, consistent with a “chronification” concept rather than expecting complete eradication from a single line of therapy.
In parallel, our recent translational focus addresses a second, complementary resistance principle in brain tumours: multicellular connectivity. Building on the concept that glioma cells form therapy-resistant networks, we pursue the Alcatraz Strategy—a multimodal roadmap to physically and functionally isolate tumour cells, analogous to preventing coordinated escape and repair. This framework combines (i) advanced neurosurgical approaches such as supramarginal resection targeting infiltrative network zones beyond the enhancing mass, with (ii) morphological network disruption (e.g., Meclofenamate to impair tumour microtube formation/outgrowth), and (iii) functional disconnection, including inhibition of gap-junction–mediated coupling and reduction of excitatory neuronal input into the malignant network.
These approaches are tightly embedded in our long-term collaborations with the Department of Neurosurgery, where the surgical and translational neuro-oncology programs provide the clinical and experimental backbone for implementing and testing such concepts—from refined resection strategies to perioperative pharmacological add-ons and trial-driven biomarker readouts of network disruption and tumour adaptation.
Our group runs an active science-outreach program that aims to bring biomedical research closer to the public—especially to children and adolescents—by making complex topics tangible, interactive, and relevant to everyday life. Building on our work on misinformation and the observation that young people and parents are specifically targeted by anti-science narratives, we translate core scientific skills (e.g., source evaluation, separating facts from opinions, understanding experimental design, and updating beliefs based on evidence) into age-appropriate, engaging formats.



We go out to schools, organise exhibitions and give talks to the interested public
Our research on glioblastoma was financially supported by:
- Förderkreis für tumor- und leukämiekranke Kinder Ulm e.V. (local charity)
- International Graduate School in Molecular Medicine Ulm (State of Baden-Württemberg)
Here is a selection of publications related to our glioblastoma focus:
2025: Vogler, M., Braun, Y., Smith, V. M., Westhoff, M. A., Pereira, R. S., Pieper, N. M., Anders, M., Callens, M., Vervliet, T., Abbas, M., Macip, S., Schmid, R., Bultynck, G. and Dyer, M. J. "The BCL2 family: from apoptosis mechanisms to new advances in targeted therapy." Signal Transduct Target Ther 10(1):
2024: Ongemach, E., Zerrinius, D., Heimann, P., Wirtz, C. R., Debatin, K.-M., Westhoff, M. A. and Peraud, A. "The Potential Role of the Extracellular Matrix Glycoprotein Reelin in Glioblastoma Biology." Pharmaceuticals 17(3): 401.
2024: Schneider, M., Potthoff, A.-L., Karpel-Massler, G., Schuss, P., Siegelin, M. D., Debatin, K.-M., Duffau, H., Vatter, H., Herrlinger, U. and Westhoff, M. A. "The Alcatraz-Strategy: a roadmap to break the connectivity barrier in malignant brain tumours." Molecular Oncology 18(12): 2890-2905.
2023: Westhoff, M. A., Posovszky, C. and Debatin, K.-M. "How to Respond to Misinformation From the Anti-Vaccine Movement." INQUIRY: The Journal of Health Care Organization, Provision, and Financing 60: 00469580231155723.
2022: Westhoff, M. A., Schuler-Ortoli, M., Zerrinius, D., Hadzalic, A., Schuster, A., Strobel, H., Scheuerle, A., Wong, T., Wirtz, C. R., Debatin, K.-M. and Peraud, A. "Bcl-XL but Not Bcl-2 Is a Potential Target in Medulloblastoma Therapy." Pharmaceuticals 15(1): 91.
2022: Konig, S., Strobel, H., Grunert, M., Lyszkiewicz, M., Brühl, O., Karpel-Massler, G., Ziętara, N., La Ferla-Brühl, K., Siegelin, M. D., Debatin, K.-M. and Westhoff, M. A. "Unblinding the watchmaker: cancer treatment and drug design in the face of evolutionary pressure." Expert Opinion on Drug Discovery 17(10): 1081-1094.
2021: Kattner, P., Zeiler, K., Herbener, V. J., Ferla-Brühl, K. L., Kassubek, R., Grunert, M., Burster, T., Brühl, O., Weber, A. S., Strobel, H., Karpel-Massler, G., Ott, S., Hagedorn, A., Tews, D., Schulz, A., Prasad, V., Siegelin, M. D., Nonnenmacher, L., Fischer-Posovszky, P., Halatsch, M.-E., Debatin, K.-M. and Westhoff, M. A. "What Animal Cancers teach us about Human Biology." Theranostics 11(14): 6682-6702.
2021: Halatsch, M.-E., Kast, R. E., Karpel-Massler, G., Mayer, B., Zolk, O., Schmitz, B., Scheuerle, A., Maier, L., Bullinger, L., Mayer-Steinacker, R., Schmidt, C., Zeiler, K., Elshaer, Z., Panther, P., Schmelzle, B., Hallmen, A., Dwucet, A., Siegelin, M. D., Westhoff, M. A., Beckers, K., Bouche, G. and Heiland, T. "A phase Ib/IIa trial of 9 repurposed drugs combined with temozolomide for the treatment of recurrent glioblastoma: CUSP9v3." Neuro-Oncology Advances 3(1).
2021: Schneider, M., Vollmer, L., Potthoff, A.-L., Ravi, V. M., Evert, B. O., Rahman, M. A., Sarowar, S., Kueckelhaus, J., Will, P., Zurhorst, D., Joseph, K., Maier, J. P., Neidert, N., d’Errico, P., Meyer-Luehmann, M., Hofmann, U. G., Dolf, A., Salomoni, P., Güresir, E., Enger, P. Ø., Chekenya, M., Pietsch, T., Schuss, P., Schnell, O., Westhoff, M. A., Beck, J., Vatter, H., Waha, A., Herrlinger, U. and Heiland, D. H. "Meclofenamate causes loss of cellular tethering and decoupling of functional networks in glioblastoma." Neuro-Oncology 23(11): 1885-1897.
2019: Kattner, P., Strobel, H., Khoshnevis, N., Grunert, M., Bartholomae, S., Pruss, M., Fitzel, R., Halatsch, M. E., Schilberg, K., Siegelin, M. D., Peraud, A., Karpel-Massler, G., Westhoff, M. A. and Debatin, K. M. "Compare and contrast: pediatric cancer versus adult malignancies." Cancer Metastasis Rev 38(4): 673-682.