AG Bergmann - Immunomonitoring in Trauma and Sepsis
Christian graduated from the Medical Faculty of the Technical University of Munich, Germany. He initiated his scientific career by obtaining his doctoral degree revealing reciprocal activation of Regulatory T cell and platelet interaction in trauma. He began his clinical career at the Department of Trauma Surgery, University Hospital Klinikum rechts der Isar, Technical University of Munich. He successfully applied for a full-time scholarship from the German Research Foundation (DFG) which funded his postdoctoral fellowship at the University of Cincinnati, Ohio, USA. The main focus of his work was to characterize immunosuppression in murine and human trauma and sepsis via IL-10 and functional IFN-gamma response. Returning to Germany he established his working-group in September 2021.
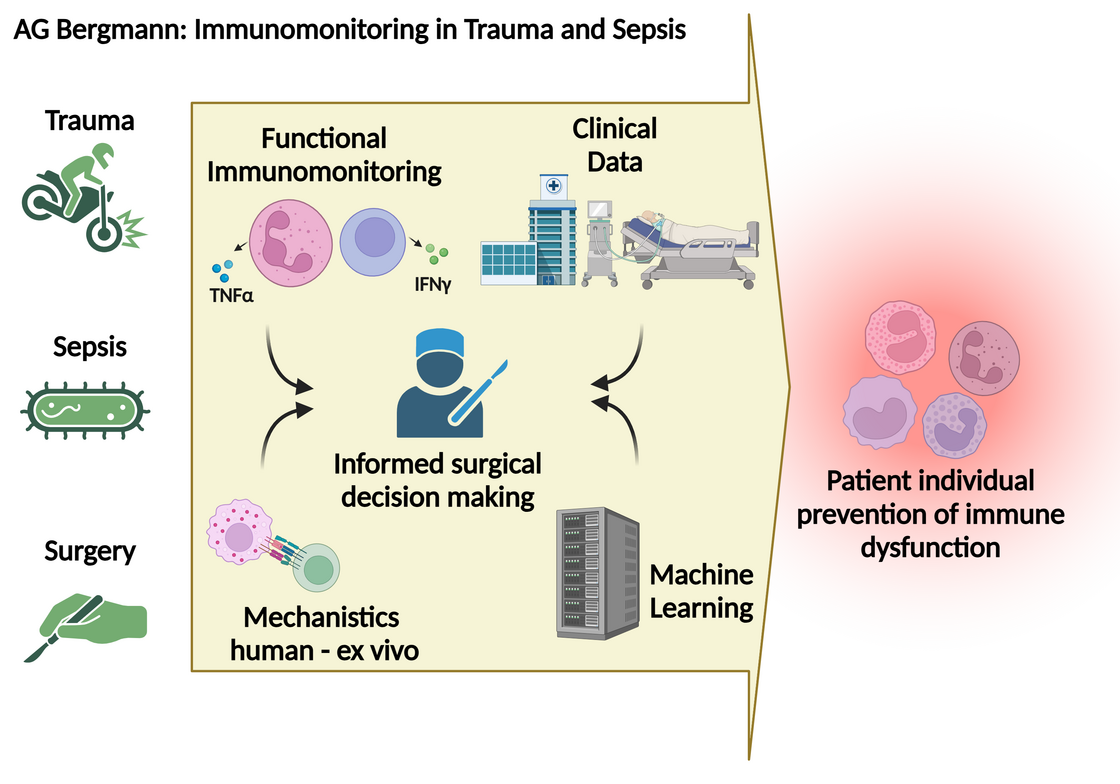
Christian is currently working both in surgical clinical care and in the laboratory as a clinician scientist. He applies functional immunomonitoring focusing on adaptive immunity in translational clinical studies to characterize immune dysfunction in trauma and sepsis patients to predict patient-individual outcomes. Moreover, his research group in Ulm is deploying automated and machine-learning data analyses to predict adverse effects of surgical and non-surgical care in sepsis and trauma patients.
Keywords Trauma, Sepsis, Machine Learning, Functional Immunonomitoring, Chronic Critical Illness
Start Up funding SFB 1149
SFB 1149 Start-up Förderung Sonderforschungsbereich 1149, 09/2022
Clinician Scientist Programm Basic Clinician Scientist Programm (Med. Fakultät Universität Ulm), seit 07/2022
SFB 1149 Start-up Förderung Sonderforschungsbereich 1149, 01/2022
SFB 1149 Anschubfinanzierung Sonderforschungsbereich 1149, 12/2021
Forschungsstipendium Forschungsstipendium der DFG, 05/2019 – 08/2021
Reichardt LM, Hindelang B, Süberkrüb L, Hamberger KL, Graw JA, Schuetze K, Zechendorf E, Mannes M, Halbgebauer R, Wohlgemuth L, Gebhard F, Huber-Lang M, Relja B, Bergmann CB. Absolute lymphocyte count trajectory predicts clinical outcome in severely injured patients. Eur J Trauma Emerg Surg. 2025 May 2;51(1):190. doi: 10.1007/s00068-025-02864-0. PMID: 40314767; PMCID: PMC12048453.
Jooss T, Maier K, Reichardt LM, Hindelang B, Süberkrüb L, Hamberger KL, Bülow JM, Schuetze K, Gebhard F, Mannes M, Halbgebauer R, Wohlgemuth L, Huber-Lang M, Relja B, Bergmann CB. Dynamic functional assessment of T cells reveals an early suppression correlating with adverse outcome in polytraumatized patients. Front Immunol. 2025 Mar 24;16:1538516. doi: 10.3389/fimmu.2025.1538516. PMID: 40196124; PMCID: PMC11973370.
Sturm R, Haag F, Bergmann CB, Marzi I, Relja B. Alcohol drinking leads to sex-dependent differentiation of T cells. Eur J Trauma Emerg Surg. 2025 Jan 27;51(1):87. doi: 10.1007/s00068-024-02732-3. PMID: 39870931; PMCID: PMC11772406.
Price AD, Becker ER, Barrios EL, Mazer MB, McGonagill PW, Bergmann CB, Goodman MD, Gould RW, Rao M, Polcz VE, Kucaba TA, Walton AH, Miles S, Xu J, Liang M, Loftus TJ, Efron PA, Remy KE, Brakenridge SC, Badovinac VP, Griffith TS, Moldawer LL, Hotchkiss RS, Caldwell CC. Surviving septic patients endotyped with a functional assay demonstrate active immune responses. Front Immunol. 2024 Oct 14;15:1418613. doi: 10.3389/fimmu.2024.1418613. PMID: 39469706; PMCID: PMC11513262.
Ungaro RF, Xu J, Kucaba TA, Rao M, Bergmann CB, Brakenridge SC, Efron PA, Goodman MD, Gould RW, Hotchkiss RS, Liang M, Mazer MB, McGonagill PW, Moldawer LL, Remy KE, Turnbull IR, Caldwell CC, Badovinac VP, Griffith TS. Development and optimization of a diluted whole blood ELISpot assay to test immune function. J Immunol Methods. 2024 Oct;533:113743. doi: 10.1016/j.jim.2024.113743. Epub 2024 Aug 13. PMID: 39147231; PMCID: PMC11398710.
Barrios EL, Mazer MB, McGonagill PW, Bergmann CB, Goodman MD, Gould RW, Rao M, Polcz VE, Davis RJ, Del Toro DE, Dirain ML, Dram A, Hale LO, Heidarian M, Kim CY, Kucaba TA, Lanz JP, McCray AE, Meszaros S, Miles S, Nelson CR, Rocha IL, Silva EE, Ungaro RF, Walton AH, Xu J, Zeumer-Spataro L, Drewry AM, Liang M, Bible LE, Loftus TJ, Turnbull IR, Efron PA, Remy KE, Brakenridge SC, Badovinac VP, Griffith TS, Moldawer LL, Hotchkiss RS, Caldwell CC. Adverse outcomes and an immunosuppressed endotype in septic patients with reduced IFN-γ ELISpot. JCI Insight. 2024 Jan 23;9(2):e175785. doi: 10.1172/jci.insight.175785. PMID: 38100268; PMCID: PMC10906237.
Fachet M, Mushunuri RV, Bergmann CB, Marzi I, Hoeschen C, Relja B. Utilizing predictive machine-learning modelling unveils feature-based risk assessment system for hyperinflammatory patterns and infectious outcomes in polytrauma. Front Immunol. 2023 Dec 12;14:1281674. doi:10.3389/fimmu.2023.1281674. PMID: 38193076; PMCID: PMC10773821.
Liette MD, Crisologo PA, Masadeh S, Yang SH, Bergmann CB, Caldwell CC, Henning JA. A Prospective Analysis of the SVS WIfI Classification System to Stratify Immediate and 1-Year Patient Outcomes. J Foot Ankle Surg. 2023 Feb 15:S1067-2516(23)00035-2. doi: 10.1053/j.jfas.2023.02.003. Epub ahead of print. PMID: 36933979.
Bergmann CB, McReynolds CB, Wan D, Singh N, Goetzman H, Caldwell CC, Supp DM, Hammock BD. sEH-derived metabolites of linoleic acid drive pathologic inflammation while impairing key innate immune cell function in burn injury. Proc Natl Acad Sci U S A. 2022 Mar 29;119(13):e2120691119. doi: 10.1073/pnas.2120691119. Epub 2022 Mar 21. PMID: 35312372; PMCID: PMC9060469.
Schuermann LE, Bergmann CB, Goetzman H, Caldwell CC, Satish L. Heat-killed probiotic Lactobacillus plantarum affects the function of neutrophils but does not improve survival in murine burn injury. Burns. 2022 Jun 30:S0305-4179(22)00164-4. doi: 10.1016/j.burns.2022.06.015. Epub ahead of print. PMID: 35850881.
Salyer CE, Bergmann CB, Hotchkiss RS, Crisologo PA, Caldwell CC. Functional Characterization of Neutrophils Allows Source Control Evaluation in a Murine Sepsis Model. J Surg Res. 2022 Jun;274:94-101. doi: 10.1016/j.jss.2021.12.037. Epub 2022 Feb 5. PMID: 35134595; PMCID: PMC9038647.
Bock M, Bergmann CB, Jung S, Biberthaler P, Heimann L, Hanschen M. Platelets differentially modulate CD4+ Treg activation via GPIIa/IIIb-, fibrinogen-, and PAR4-dependent pathways. Immunol Res. 2022 Apr;70(2):185-196. doi: 10.1007/s12026-021-09258-5. Epub 2021 Dec 21. PMID: 34932195; PMCID: PMC8917040.
Rupp MC, Bergmann CB, Jung S, Bock M, Biberthaler P, Heimann L, Hanschen M. The posttraumatic response of CD4+ regulatory T cells is modulated by direct cell-cell contact via CD40L- and P-selectin-dependent pathways. Cent Eur J Immunol. 2021;46(3):283-294. doi: 10.5114/ceji.2021.109171. Epub 2021 Oct 19. PMID: 34764800; PMCID: PMC8574106.
Bergmann CB, Hammock BD, Wan D, Gogolla F, Goetzman H, Caldwell CC, Supp DM. TPPU treatment of burned mice dampens inflammation and generation of bioactive DHET which impairs neutrophil function. Sci Rep. 2021 Aug 16;11(1):16555. doi: 10.1038/s41598-021-96014-2. PMID: 34400718; PMCID: PMC8368302.
Bergmann CB, Beckmann N, Salyer CE, Crisologo PA, Nomellini V, Caldwell CC. Lymphocyte Immunosuppression and Dysfunction Contributing to Persistent Inflammation, Immunosuppression, and Catabolism Syndrome (PICS). Shock. 2021 Jun 1;55(6):723-741. doi: 10.1097/SHK.0000000000001675. PMID: 33021569.
Bergmann CB, Beckmann N, Salyer CE, Hanschen M, Crisologo PA, Caldwell CC. Potential Targets to Mitigate Trauma- or Sepsis-Induced Immune Suppression. Front Immunol. 2021 Feb 25;12:622601. doi: 10.3389/fimmu.2021.622601. PMID: 33717127; PMCID: PMC7947256.
Salyer CE, Bomholt C, Beckmann N, Bergmann CB, Plattner CA, Caldwell CC. Novel Therapeutics for the Treatment of Burn Infection. Surg Infect (Larchmt). 2021 Feb;22(1):113-120. doi: 10.1089/sur.2020.104. Epub 2020 May 19. PMID: 32429749; PMCID: PMC7826423.
Bergmann CB, Salyer CE, Beckmann N, Caldwell CC. Intraperitoneal Neutrophil IL-10 production is promoted by interferon γ in a murine model of sepsis model in the acute phase of sepsis. Biochem Biophys Res Commun. 2020 Sep 10;530(1):278-284. doi: 10.1016/j.bbrc.2020.07.089. Epub 2020 Aug 6. PMID: 32828299; PMCID: PMC7597436.
Bock M, Bergmann CB, Jung S, Kalbitz M, Relja B, Huber-Wagner S, Biberthaler P, van Griensven M, Hanschen M. The posttraumatic activation of CD4+ T regulatory cells is modulated by TNFR2- and TLR4-dependent pathways, but not by IL-10. Cell Immunol. 2018 Sep;331:137-145. doi: 10.1016/j.cellimm.2018.06.009. Epub 2018 Jun 22. PMID: 29954581.
Bergmann CB, Hefele F, Unger M, Huber-Wagner S, Biberthaler P, van Griensven M, Hanschen M. Platelets modulate the immune response following trauma by interaction with CD4+ T regulatory cells in a mouse model. Immunol Res. 2016 Apr;64(2):508-17. doi: 10.1007/s12026-015-8726-1. PMID: 26471021.
Team
"At the crossroads of trauma and immunity, my research seeks to unravel the body’s earliest responses to injury - so we can transform critical moments into better outcomes."
“I am passionate about improving outcomes for trauma patients by understanding how their immune system responds to severe injury.”
"My interest in research and the desire to help others motivate me to do this experimental doctoral thesis in order to make an active contribution during my studies."
"Small cell - big impact: By studying the interactions between polytrauma and immune dysfunction, I hope to contribute to new therapeutic approaches and improved outcomes."
"Driven by the urgent need to improve outcomes in sepsis, I am committed to deepening our understanding of the underlying immune responses."
„My work aims to help bring new insights in diagnosis and therapy of trauma patients "from bench to bedside”
"As a doctoral candidate in trauma research, I aim to better understand the consequences of trauma in order to find ways to optimize its management. My research is focused on predicting and optimizing the outcomes for those affected by trauma at an early stage."
"My interest in research and passion for trauma surgery intersect in the field of immunological trauma research. Through research, I am able to expand my knowledge, contribute to the development of new innovative methods, and explore new avenues for medicine every day."
"I would like to specialize in a traumatology field after my studies and am using my doctoral thesis to gain a deeper insight and a different perspective on the subject."










