Our group focusses on two areas of research:
A. The CK1 (casein kinase 1) family: functions, regulatory mechanisms, its importance in tumorigenesis and tumor progression, and its potential as drug target for the treatment of cancer and neurodegenerative diseases
B. Obesity: Pathophysiology and its influence on the immune system resulting in - potentially irreversible - modifications
Ad A: The CK1 (casein kinase 1) family: functions, regulatory mechanisms, its importance in tumorigenesis and tumor progression, and its potential as drug target for the treatment of cancer and neurodegenerative diseases
The CK1 family is a distinct phylogenetic family of highly conserved, monomeric, second messenger-independent serine/threonine-specific kinases, which are ubiquitously expressed. In mammalian cells, seven CK1 isoforms (α, β, γ1-3, δ, and ε) and several splice variants of CK1 isoforms have been identified to date, all of which show high homology in their kinase domains but differ in their regulatory C-terminal domains. CK1 family members play important roles in normal cell physiology by modulating the functions of key regulatory proteins by site-specific phosphorylation. Deregulation of the expression and/or activity of CK1 isoforms was observed in various disorders among them neurodegenerative diseases and cancer. Within the last decade several CK1-specific small molecule inhibitors (SMIs) have been developed showing promising therapeutic potential for the treatment of neurodegenerative diseases and cancer. However, it remains challenging to develop isoform-specific inhibitors due to the high homology between CK1 isoforms, especially between CK1δ and CK1ε, and to achieve high solubility and cell permeability of SMIs without losing CK1 isoform specificity.
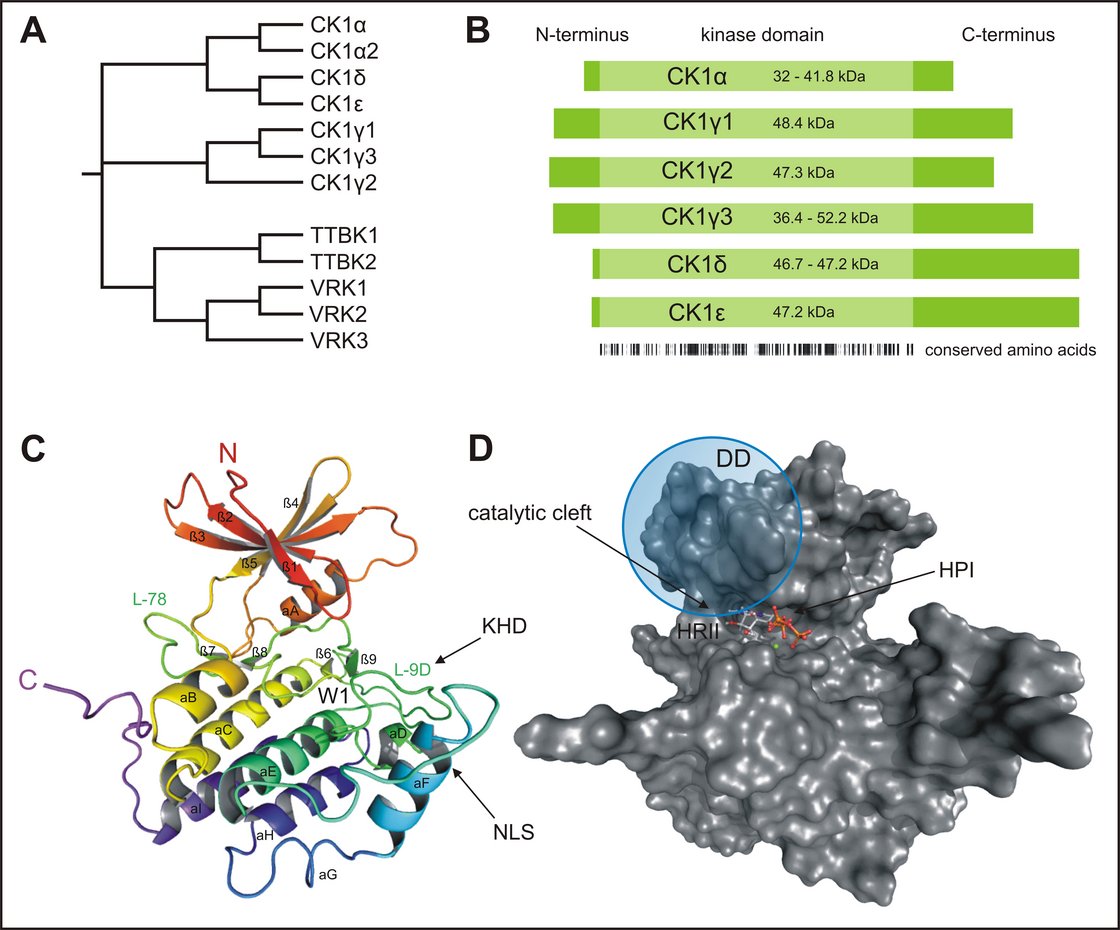
CK1 family and three-dimensional structure of CK1δ
(A) Phylogenetic three of CK1 superfamily. (B) CK1 isoforms and their kinase domain (KD), N-, and C-terminal domains. Conserved amino acids are shown. CK1δ is shown in its (C) ribbon or (D) surface representation. (C) The N-terminal lobe is characterized by mostly β-sheets, while the C-terminal lobe by α-helics. Different domains are shown, such as the kinesin homology domain (KHD) and its and the L-9D loop, as well as the putative nuclear localization sequence (NLS). (D) Here the catalytic cleft, the dimerization domain (DD), the hydrophobic pocket I (HPI), the hydrophobic region II (HRII) are shown. Moreover, the binding of a small molecule inhibitor in the ATP-binding pocket is shown. Knippschild et al. 2014, Front Oncol, 2014. 4: p. 96 Figure 1. Copyright license: CC BY-NC 3.0; creativecommons.org/licenses/by/3.0
Ad B. Obesity: Pathophysiology and its influence on the immune system resulting in - potentially irreversible - modifications
Obesity is a worldwide epidemic. The excessive accumulation of adipose tissue leads to the development of severe comorbidities including insulin resistance, type 2 diabetes mellitus, hepatic steatosis, cardiovascular diseases including hypertension and atherosclerosis, as well as an increased risk of developing certain types of cancer. Conventional therapy concepts involving e.g. diet, physical exercise, or behavior therapy often fail. As a consequence of increasing obesity, the number of bariatric interventions is also rapidly increasing. These interventions are essential for the health of patients, since in most cases it is not feasible to significantly reduce the accumulated excess weight through conservative therapy. It has been proven that bariatric surgery can reduce the accumulated excess weight by 60-80% (excess weight loss - EWL) within 12 months in the majority of patients.
Trauma ranks as the primary cause of mortality in the population under the age of 40 years. 20-25% of these deadly traumas include thoracic injuries which makes this the third frequent cause of trauma-related death. Additionally, the rate of mortality rises in the coincidence of extra-thoracic injuries. These types of traumas are described as polytrauma or combined trauma and are defined as injuries of various regions of the body at the same time, whereas one of those injuries is life threatening. A common reason for this kind of combination of injuries are severe accidents, such as car crashes. 20% of those polytraumatic injuries end lethal.
The efficiency of treatment and the life expectancy after polytraumatic injuries is highly influenced by additional risk factors such as obesity. Obesity is an increased risk factor for several diseases and can influence regeneration processes in a negative manner, triggered by a state of chronic inflammation in obese individuals. In addition to an excessive fat accumulation it was shown that the composition of adipose tissue resident cells changes, finally leading to an alternated profile of production and secretion of pro- and anti-inflammatory cytokines. It has been shown in previous work, that obesity can influence the healing process after a traumatic injury in mice.
Key areas of our CK1-related research:
Members of the CK1 family represent central components in the regulation of several cellular functions linked to cell cycle progression, spindle dynamics, chromosome segregation and vesicle transport processes. In interphase cells CK1δ co-localizes with the Golgi apparatus and the trans Golgi network (TGN). Furthermore, CK1δ associates with vesicles mediating the transport between the endoplasmatic reticulum (ER) and the Golgi apparatus as well as with TGN-derived vesicles as well as vinteracts with microtubules in interphase cells.
During mitosis CK1δ is associated to the spindle apparatus thereby modulating microtubules by phosphorylation of α-, β-, and γ-tubulin, thereby exerting stress induced functions at the mitotic spindle and centrosome. In addition to the direct, The polymerization and stability of microtubules is not only regulated by direct interaction of CK1 with microtubules, it can also be regulated by CK1-mediated modulation of microtubule associated proteins (MAPs). It has been shown that CK1δ regulates microtubule- and spindle-dynamics in response to genotoxic stress thereby ensuring genomic stability by site-specific phosphorylation of stathmin, and the MAPs MAP4, MAP1A, tau and Sid4 that delays cytokinesis. An abnormal hyperphosphorylation of tau by CK1δ can lead to microtubule destabilization and is associated with the pathogenesis of Alzheimer’s disease.
A particular interesting role of centrosome-associated CK1 has been proposed in regulating cell cycle progression by interaction with the Wnt pathway and p53. The hypothesis of CK1 fulfilling regulatory roles at the centrosome is further supported by the fact that CK1δ and ε are anchored at the centrosome through interaction with AKAP450. Interestingly there is evidence that a subpopulation of p53 is located at the centrosome thereby preventing genomic instability. Therefore, the coordinated function of both CK1 and p53 could ensure the integrity of the centrosome and genomic stability.
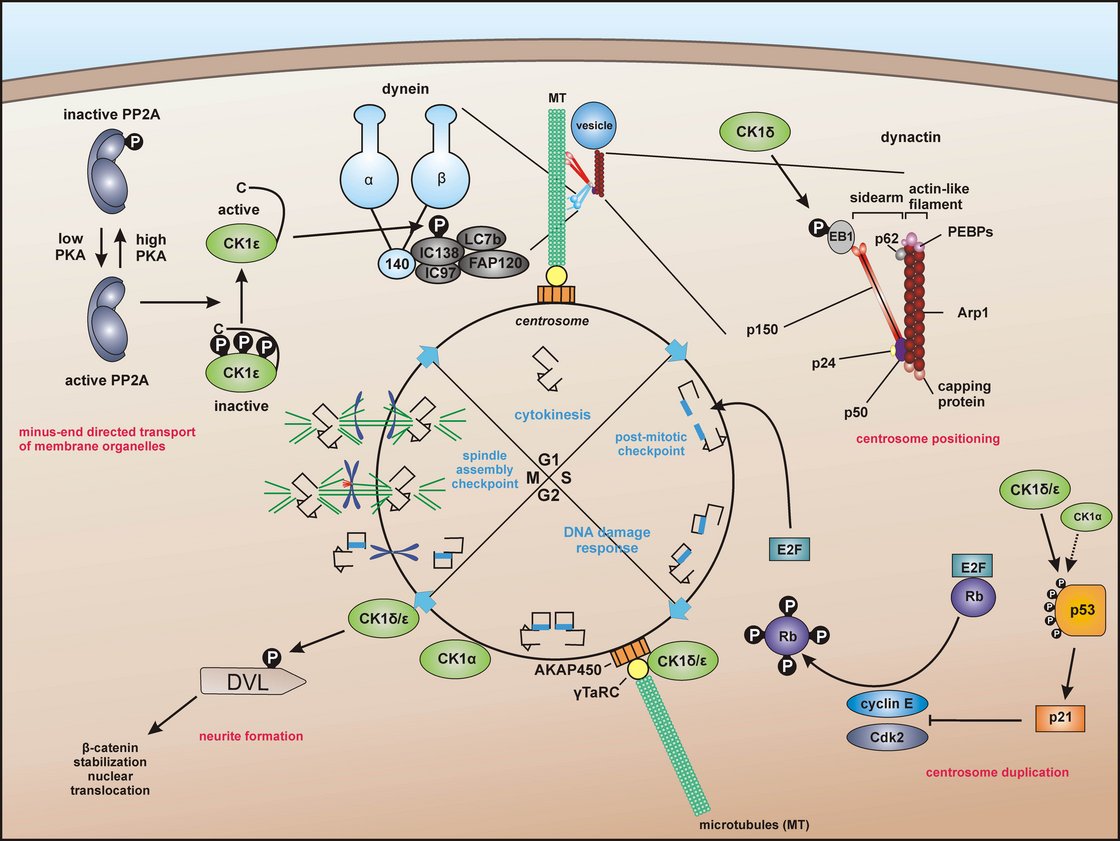
Centrosome-associated functions of CK1
For dynein–dependent transport along microtubules, CK1ε phosphorylates the dynein intermediate chain (DIC) of dynein, likely IC138, thereby activating minus-end directed transport of membrane organelles along microtubules (MT). CK1δ and CK1ε are associated with the centrosome via interaction with the scaffold protein AKAP450 (A-kinase anchor protein 450). Both isoforms are related to Wnt-signaling and neurite outgrowth by phosphorylation of DVL. In addition, CK1δ phosphorylates end binding protein 1 (EB1) which is relevant for centrosome positioning during T-cell activation. Furthermore, a subpopulation of p53 in coordinated function with CK1 at the centrosome could ensure the integrity of the centrosome and thereby maintain genomic stability. Knippschild et al. 2014, Front Oncol, 2014. 4: p. 96 Figure 3. Copyright license: CC BY-NC 3.0; creativecommons.org/licenses/by/3.0/.
- Behrend L, Milne DM, Stöter M, Deppert W, Campbell LE, Meek DW, Knippschild U. 2000. IC261, a specific inhibitor of the protein kinases casein kinase 1-delta and -epsilon, triggers the mitotic checkpoint and induces p53-dependent postmitotic effects. Oncogene 19:5303-13.
- Behrend L, Stoter M, Kurth M, Rutter G, Heukeshoven J, Deppert W, Knippschild U. 2000. Interaction of casein kinase 1 delta (CK1delta) with post-Golgi structures, microtubules and the spindle apparatus. Eur J Cell Biol 79:240-51.
- Stöter M, Bamberger AM, Aslan B, Kurth M, Speidel D, Loning T, Frank HG, Kaufmann P, Lohler J, Henne-Bruns D, Deppert W, Knippschild U. 2005. Inhibition of casein kinase I delta alters mitotic spindle formation and induces apoptosis in trophoblast cells. Oncogene 24:7964-75.
- Wolff S, Xiao Z, Wittau M, Süssner N, Stöter M, Knippschild U. 2005. Interaction of casein kinase 1 delta (CK1 delta) with the light chain LC2 of microtubule associated protein 1A (MAP1A). Biochim Biophys Acta 1745:196-206.
- Wolff S, Stöter M, Giamas G, Piesche M, Henne-Bruns D, Banting G, Knippschild U. 2006. Casein kinase 1 delta (CK1delta) interacts with the SNARE associated protein snapin. FEBS Lett 580:6477-84.
- Stöter M, Krüger M, Banting G, Henne-Bruns D, Knippschild U. 2014. Microtubules depolymerization caused by the CK1 inhibitor IC261 may be not mediated by CK1 blockage. PLoS One 9:e100090.
- Krüger M, Kalbacher H, Kastritis PL, Bischof J, Barth H, Henne-Bruns D, Vorgias C, Sarno S, Pinna LA, Knippschild U. 2016. New potential peptide therapeutics perturbing CK1delta/alpha-tubulin interaction. Cancer Lett 375:375-383.
CK1δ expression and activity needs to be strictly controlled on different levels because of its ubiquitous expression and to ensure proper function in regulating several important cellular signal transduction pathways. CK1δ activity can be increased as a consequence of various stimuli as well as cellular stress. Apart from transcriptional or translational regulation of CK1δ protein expression, its activity can also be modulated at protein level by sequestration to particular subcellular compartments, interaction with other proteins, dimerization, and posttranslational modifications. Reversible, site-specific phosphorylation by intramolecular autophosphorylation or site-specific phosphorylation by upstream cellular kinases is an important mechanism to fine-tune CK1δ activity. Generally, CK1δ kinase activity is reduced upon (C-terminal) phosphorylation and can be modulated by the action of kinases and phosphatases in vivo. Our research within the last years focused on the identification of cellular kinases phosphorylating CK1δ and on the characterization of the physiological consequences of site-specific phosphorylation. Within the C-terminal domain of CK1δ we identified the phosphorylation sites for several kinases, among them protein kinase A (PKA), protein kinase B (Akt), CDC-like kinase 2 (CLK2), cyclin-dependent kinases (CDK2/E and CDK5/p35), protein kinase C α (PKCα), and checkpoint kinase 1 (Chk1). CK1δ kinase activity was enhanced after mutation of the PKA phosphorylation site Ser370 to alanine (S370A) and the key role of Ser370 was furthermore underlined in vivo by microinjection of the CK1δ S370A mutant in Xenopus laevis embryos, which resulted in an enhanced formation of an ectopic dorsal axis in Xenopus embryos. Furthermore, incubation of CK1δ together with Chk1, PKCα, CDK2/E, or CDK5/p35 resulted in reduction of CK1δ mediated substrate phosphorylation in vitro. However, there is also evidence from cell culture-based analyses demonstrating inhibitory effects of site-specific phosphorylation on CK1δ kinase activity.
While most studies focused on the identification of phosphorylation sites in the C-terminal domain, we have started to identify phosphorylation sites for cellular kinases within the kinase domain. Key areas of further research are (i) identification of additional cellular kinases able to phosphorylate CK1δ within the kinase domain, (ii) characterization of site-specific phosphorylation on CK1δ activity and its physiological functions as well as characterization of the effects of site-specific phosphorylation on the effectivity of small molecule inhibitors.
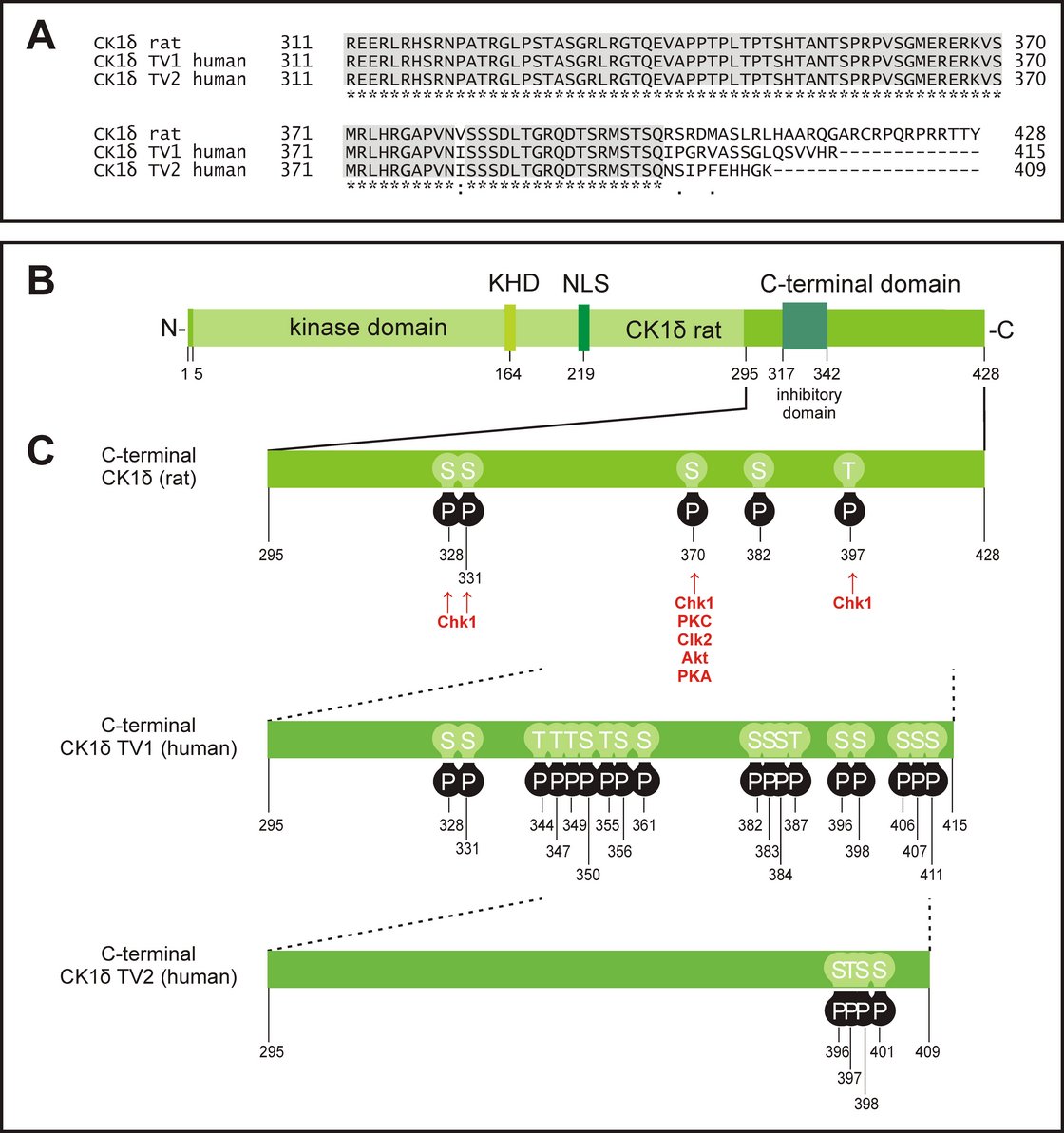
Phosphorylation sites located in the C-terminal domain of CK1δ
(A) Alignment of the rat CK1δ C-terminal sequence with the human CK1δ transcription variants 1 and 2 C-terminal sequences (accession numbers L07578, NM001893, and NM139062, respectively) generated by using the program ClustalW (370, 371), showing conserved amino acids (gray) and obvious differences in the C-terminal domain beyond amino acid 399. (B) Domain structure of human CK1δ (NLS: nuclear localization signal, KHD: kinesin homology domain). (C) Phosphorylation sites in the C-terminal regulatory domains of CK1δ rat and human transcription variants 1 and 2, that have so far been confirmed experimentally (187, 188, 372-385). Kinases identified for phosphorylation of the C-terminal domain are shown for rat CK1δ (187, 188). Knippschild et al. 2014, Front Oncol, 2014. 4: p. 96 Figure 2. Copyright license: CC BY-NC 3.0;
creativecommons.org/licenses/by/3.0/.
- Böhm T, Meng Z, Haas P, Henne-Bruns D, Rachidi N, Knippschild U, Bischof J. 2019. The kinase domain of CK1delta can be phosphorylated by Chk1. Biosci Biotechnol Biochem 83:1663-1675.
- Meng Z, Böhm T, Xu P, Henne-Bruns D, Peifer C, Witt L, Knippschild U, Bischof J. 2019. Kinase activity of casein kinase 1 delta (CK1delta) is modulated by protein kinase C alpha (PKCalpha) by site-specific phosphorylation within the kinase domain of CK1delta. Biochim Biophys Acta Proteins Proteom 1867:710-721.
- Ianes C, Xu P, Werz N, Meng Z, Henne-Bruns D, Bischof J, Knippschild U. 2016. CK1delta activity is modulated by CDK2/E- and CDK5/p35-mediated phosphorylation. Amino Acids 48:579-92.
- Meng Z, Bischof J, Ianes C, Henne-Bruns D, Xu P, Knippschild U. 2016. CK1delta kinase activity is modulated by protein kinase C alpha (PKCalpha)-mediated site-specific phosphorylation. Amino Acids 48:1185-97.
- Bischof J, Randoll SJ, Süssner N, Henne-Bruns D, Pinna LA, Knippschild U. 2013. CK1delta kinase activity is modulated by Chk1-mediated phosphorylation. PLoS One 8:e68803.
- Giamas G, Hirner H, Shoshiashvili L, Grothey A, Gessert S, Kühl M, Henne-Bruns D, Vorgias CE, Knippschild U. 2007. Phosphorylation of CK1delta: identification of Ser370 as the major phosphorylation site targeted by PKA in vitro and in vivo. Biochem J 406:389-98.
Cancer-associated functions of CK1δ are closely linked to the regulatory roles of CK1δ in Wnt/β-catenin-, p53-, Hedgehog-, and Hippo-related signaling. Deregulation of CK1δ expression and/or activity as well as the occurrence of mutations within the coding region of CK1δ resulting in increased kinase activity and oncogenic potential have been described in various tumor entities, including among others, gastrointestinal tumors, breast cancer, kidney cancer, hematological malignancies, and skin cancer. Pharmacological inhibition or silencing of CK1 often results in reduced cell viability, growth, proliferation, migration, metastasis, and tumor regression. Moreover, in colorectal cancer (CRC) patients low CK1δ expression levels correlates with prolonged survival rates. CK1δ mutations can also influence CK1δ activity and may have an impact on tumor development and/or progression. In this context, we could show that the activity and tumorigenic potential of CK1δT67S is increased compared to wild-type CK1δ in tissue culture and xenografts. Furthermore, we could show decreased activity of CK1δ in SV40-transformed cells in vitro and in SV40-induced mammary carcinogenesis in a transgenic mouse model for breast cancer (ductal carcinoma in situ, DCIS) in vivo. In this model CK1δ kinase activity is impaired by site-specific mutations (e.g. N172D) and transgenic, mutant CK1δ exercises a dominant-negative effect on endogenous CK1δ finally resulting in partial inhibition of CK1δ activity, decelerated tumor progression, and prolonged survival of WAP-mutCK1δ/WAP-SV40 T-Ag bitransgenic mice compared to WAP-SV40 T-Ag transgenic mice.
At present our research focus on the identification and characterization of new transformation-relevant CK1δ interaction partners as well as the validation of newly identified CK1δ wild-type- and mutant-specific small molecule inhibitors.
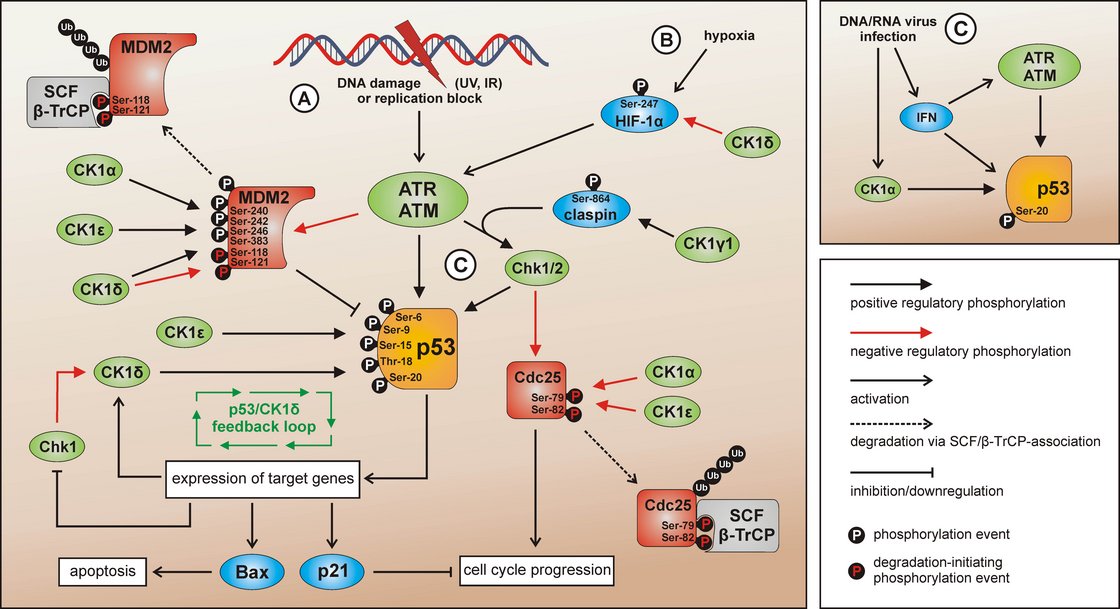
CK1 isoforms in DNA damage-induced signal transduction
After induction of DNA damage (situation A) p53 and Chk1/2 are activated by ATR/ATM-mediated phosphorylation while the p53-regulatory component MDM2 is inhibited. The activation of Chk1 is supported by claspin whereas Chk1/claspin-binding is promoted by CK1γ1-mediated phosphorylation of claspin. The CK1 isoforms α, δ, and ε are able to activate p53 by site-specific phosphorylation. Activated p53 in turn induces the expression of target genes like Bax (leading to apoptosis), p21 (leading to cell cycle arrest), and also CK1δ (autoregulatory feedback loop). MDM2-mediated degradation of p53 can be activated via interaction with and phosphorylation by CK1α, but also through phosphorylation by CK1δ or ε leading to enhanced binding of MDM2 to p53. CK1δ-mediated phosphorylation of Ser118 and Ser121 however marks MDM2 for proteasomal degradation. In case Chk1/2 gets activated after DNA damage, the phosphatase Cdc25, normally initiating cell cycle progression, is blocked by inhibitory phosphorylation and subsequent degradation. In the regulation of Cdc25 inhibition and degradation also CK1 isoforms α and ε are involved. Signaling mediated by p53 can also be initiated by hypoxia (via CK1δ-regulated HIF-1α; situation B) or DNA/RNA virus infection (via IFN and/or CK1α-related signal transduction; situation C). Depicted phosphorylation events refer to reported CK1-specific target sites. Knippschild et al. 2014, Front Oncol, 2014. 4: p. 96 Figure 4. Copyright license: CC BY-NC 3.0; creativecommons.org/licenses/by/3.0/.
Bischof J, Kretz AL, Burster T, Henne-Bruns D, Knippschild U, Xu P. 2018. Inhibition of CK1 affects Viability and Survival of Glioblastoma Cells. Biomed J Sci & Tech Res 2.
Dolde C, Bischof J, Gruter S, Montada A, Halekotte J, Peifer C, Kalbacher H, Baumann U, Knippschild U, Suter B. 2018. A CK1 FRET biosensor reveals that DDX3X is an essential activator of CK1epsilon. J Cell Sci 131.
Richter J, Kretz AL, Lemke J, Fauler M, Werner JU, Paschke S, Leithäuser F, Henne-Bruns D, Hillenbrand A, Knippschild U. 2018. CK1alpha overexpression correlates with poor survival in colorectal cancer. BMC Cancer 18:140.
Richter J, Rudeck S, Kretz AL, Kramer K, Just S, Henne-Bruns D, Hillenbrand A, Leithäuser F, Lemke J, Knippschild U. 2016. Decreased CK1delta expression predicts prolonged survival in colorectal cancer patients. Tumour Biol 37:8731-9.
Richter J, Ullah K, Xu P, Alscher V, Blatz A, Peifer C, Halekotte J, Leban J, Vitt D, Holzmann K, Bakulev V, Pinna LA, Henne-Bruns D, Hillenbrand A, Kornmann M, Leithäuser F, Bischof J, Knippschild U. 2015. Effects of altered expression and activity levels of CK1delta and varepsilon on tumor growth and survival of colorectal cancer patients. Int J Cancer 136:2799-810.
Winkler BS, Oltmer F, Richter J, Bischof J, Xu P, Burster T, Leithäuser F, Knippschild U. 2015. CK1delta in lymphoma: gene expression and mutation analyses and validation of CK1delta kinase activity for therapeutic application. Front Cell Dev Biol 3:9.
Hirner H, Gunes C, Bischof J, Wolff S, Grothey A, Kuhl M, Oswald F, Wegwitz F, Bosl MR, Trauzold A, Henne-Bruns D, Peifer C, Leithauser F, Deppert W, Knippschild U. 2012. Impaired CK1 delta activity attenuates SV40-induced cellular transformation in vitro and mouse mammary carcinogenesis in vivo. PLoS One 7:e29709.
Brockschmidt C, Hirner H, Huber N, Eismann T, Hillenbrand A, Giamas G, Radunsky B, Ammerpohl O, Böhm B, Henne-Bruns D, Kalthoff H, Leithauser F, Trauzold A, Knippschild U. 2008. Anti-apoptotic and growth-stimulatory functions of CK1 delta and epsilon in ductal adenocarcinoma of the pancreas are inhibited by IC261 in vitro and in vivo. Gut 57:799-806.
Stöter M, Bamberger AM, Aslan B, Kurth M, Speidel D, Loning T, Frank HG, Kaufmann P, Lohler J, Henne-Bruns D, Deppert W, Knippschild U. 2005. Inhibition of casein kinase I delta alters mitotic spindle formation and induces apoptosis in trophoblast cells. Oncogene 24:7964-75.
Winter M, Milne D, Dias S, Kulikov R, Knippschild U, Blattner C, Meek D. 2004. Protein kinase CK1delta phosphorylates key sites in the acidic domain of murine double-minute clone 2 protein (MDM2) that regulate p53 turnover. Biochemistry 43:16356-64.
Behrend L, Milne DM, Stöter M, Deppert W, Campbell LE, Meek DW, Knippschild U. 2000. IC261, a specific inhibitor of the protein kinases casein kinase 1-delta and -epsilon, triggers the mitotic checkpoint and induces p53-dependent postmitotic effects. Oncogene 19:5303-13.Knippschild U, Milne DM, Campbell LE, DeMaggio AJ, Christenson E, Hoekstra MF, Meek DW. 1997. p53 is phosphorylated in vitro and in vivo by the delta and epsilon isoforms of casein kinase 1 and enhances the level of casein kinase 1 delta in response to topoisomerase-directed drugs. Oncogene 15:1727-36.
Knippschild U, Milne D, Campbell L, Meek D. 1996. p53 N-terminus-targeted protein kinase activity is stimulated in response to wild type p53 and DNA damage. Oncogene 13:1387-93.
Deppert W, Kurth M, Graessmann M, Graessmann A, Knippschild U. 1991. Altered phosphorylation at specific sites confers a mutant phenotype to SV40 wild-type large T antigen in a flat revertant of SV40-transformed cells. Oncogene 6:1931-8.
Since deregulation of CK1δ expression and activity levels contributes to the pathogenesis of cancer and neurodegenerative disorders Alzheimer’s Disease (AD), Parkinson´s Disease (PD) and Amyotrophic Lateral Sclerosis (ALS), interest in modulating the activity of CK1δ has increased enormously. Therefore, CK1 family members are interesting drug targets for therapeutic applications. However, development of CK1δ specific small molecule inhibitors (SMIs) is challenging for several reasons because of the high homology of CK1 isoforms (CK1α, γ1-3, δ, ε) with overlapping or opposed physiological and pathophysiological functions, and the influence of site-specific phosphorylation of CK1δ, especially within its C-terminal regulatory domain, on efficiency of SMIs.
We focused on the validation of benzimidazole-based CK1δ-specific inhibitors and could increase the specificity, activity, and anti-proliferative activity by introducing a difluoromethyldioxolo group on the benzimidazole. Recently, we also could show that inhibitors of Wnt production (IWPs), known to be antagonists of the Wnt pathway by preventing Wnt ligand palmitoylation through inhibition of the membrane-bound O-acyltransferase porcupine (Porcn), are able to specifically inhibit CK1δ due to structural similarities to benzimidazole-based CK1 inhibitors. Furthermore, we investigated the inhibitory potential of substituted isoxazoles with a typical vicinal pyridin-4-yl/4-F-phenyl pharmacophore, which have been originally generated and characterized as ATP-competitive inhibitors for p38α MAPK.
We now focus on the validation and biological characterization of new CK1δ-/ε- specific SMIs to identify compounds with increased physicochemical properties, including solubility and cell permeability, as well as compounds exhibiting a higher affinity to CK1δ mutants with an increased oncogenic potential.
We expect to identify highly effective and isoform-selective CK1δ isoform- and CK1δ mutant-specific inhibitors with significant therapeutic potential for novel therapeutic approaches against aggressive tumor entities.
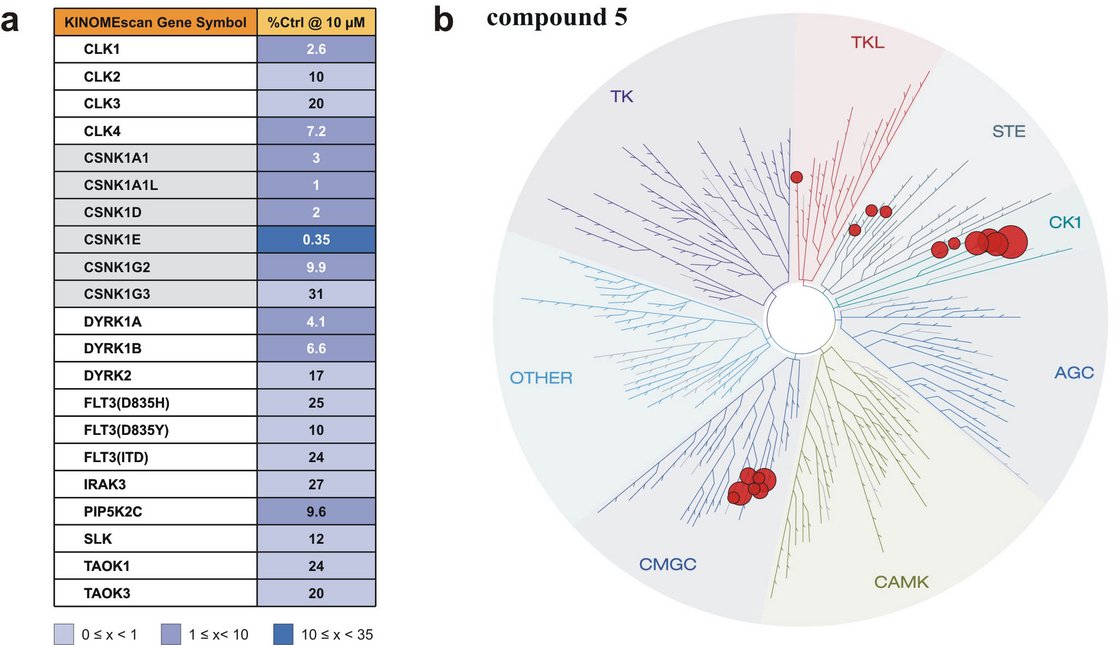
Determination of selectivity of compound 5 (Bischof et al., 2012).
In order to determine target selectivity, a panel of 442 protein kinases was screened for inhibition by compound 5 at a concentration of 10 µM. (a) Targets showing less than 35% of control activity in the presence of 5. (b) Illustration of targets phylogenetic relations listed in (a). Image generated using TREEspotTM Software Tool and reprinted with permission from KINOMEscanTM, a division of DiscoveRx Corporation, _ DISCOVERX CORPORATION 2010. “Fig. 4” published in Bischof et al. (2012) Amino Acids, used under CC BY 4.0 (http://creativecommons.org/licenses/by/4.0/).
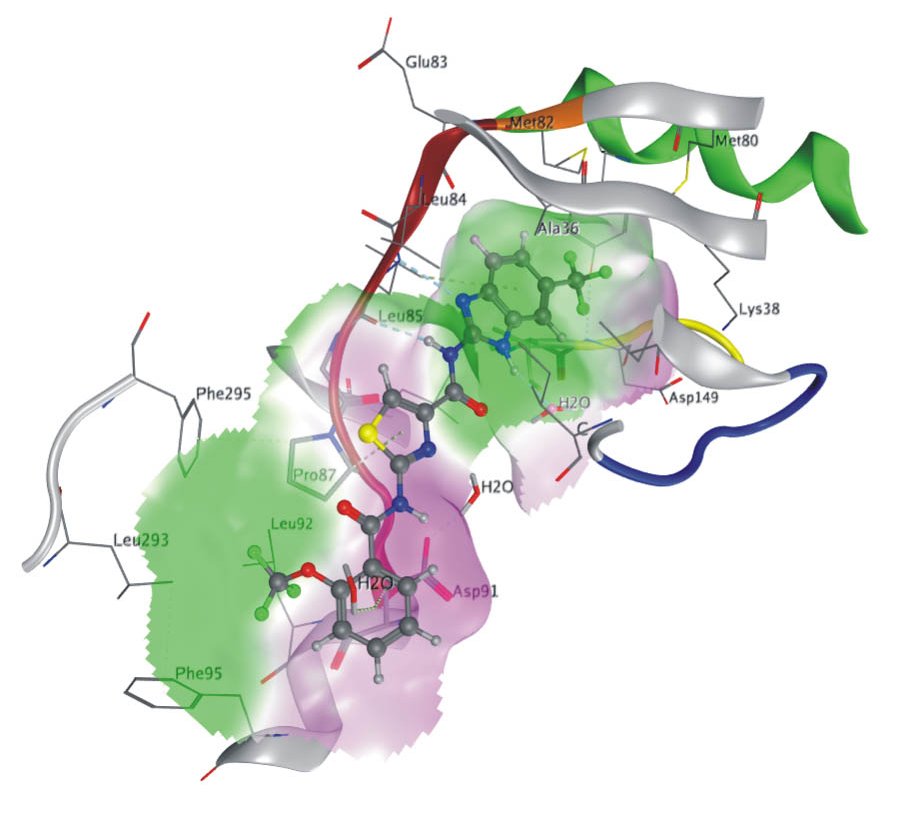
X-ray structure of compound 5 (Bischof et al., 2012) in the ATP binding pocket of CK1δ.
Residues within 4.5 Å of compound 5 are fully shown, whereas the backbone is visualized in parts, color-coded for the kinase-typical structural elements (aC green, gatekeeper orange, hinge-region red, glycine-rich loop blue and DFG-motif yellow (background)). The doted lines depict hydrogen bonds in cyan for standard and orange for p-hydrogen bonds. “Fig. 5” published in Bischof et al. (2012) Amino Acids, used under CC BY 4.0 (http://creativecommons.org/licenses/by/4.0/).
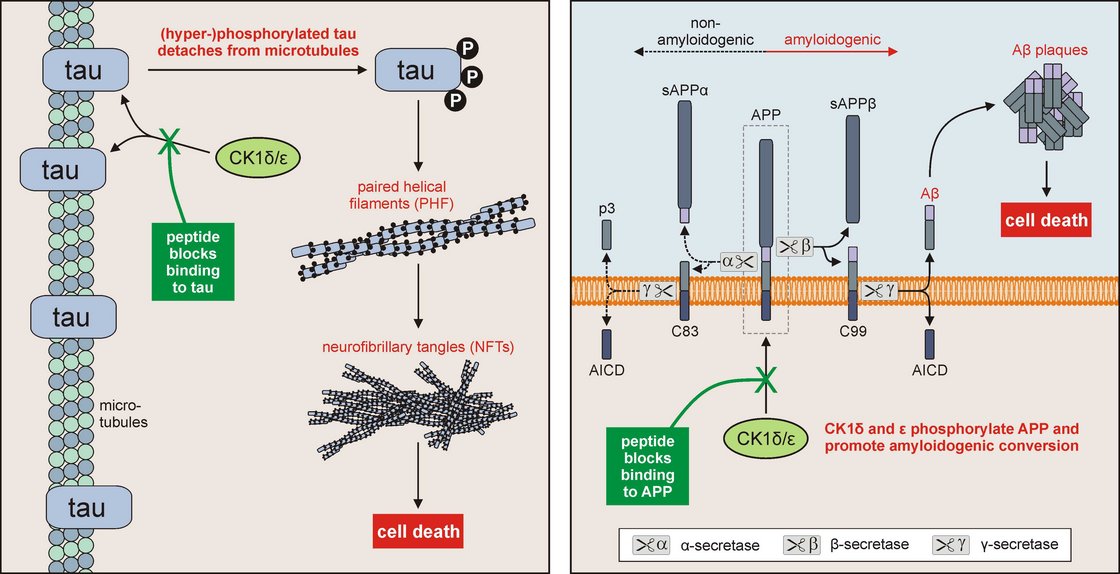
Since SMIs often show low bioavailability, off-target effects, and severe side effects, interest in identification and validation of synthetic peptides able to inhibit CK1δ kinase activity or to block the interaction of CK1δ with cellular proteins has increased in the last decade.
In this context we generated a peptide library based on the amino acid sequences of human CK1δ and ε to determine the dominant contacts of the interaction surface between α-tubulin and CK1δ. Peptide P39 (also named peptide δ-361 = peptide sequence starting at CK1δ amino acid 361) showed the strongest interaction with GST-α-tubulin. In cells, δ-361 is able to disturb the CK1δ/α-tubulin interaction, finally leading to microtubule destabilization and cell death (Kruger, Kalbacher et al. 2016).
CK1δ- and ε-derived peptides were furthermore used to determine the interaction surfaces of CK1δ and ε with its putative activating subunit DDX3X. Peptides δ-1, δ-41, and ε-41 successfully inhibited DDX3X-mediated activation of CK1δ/ε kinase activity as well as endogenous activity of CK1 in cell culture as determined by a FRET-based biosensor.This CK1δ derived library is furthermore used to determine the interactions between CK1δ and like the amyloid precursor protein (APP) and tau, which are both critically involved in the pathogenesis of Alzheimer’s disease (see project described below).
- C, Witt L, Ianes C, Bischof J, Bammert MT, Baier J, Kirschner S, Henne-Bruns D, Xu P, Kornmann M, Peifer C, Knippschild U. 2019. Newly Developed CK1-Specific Inhibitors Show Specifically Stronger Effects on CK1 Mutants and Colon Cancer Cell Lines. Int J Mol Sci 20.
- Luxenburger A, Schmidt D, Ianes C, Pichlo C, Krüger M, von Drathen T, Brunstein E, Gainsford GJ, Baumann U, Knippschild U, Peifer C. 2019. Design, Synthesis and Biological Evaluation of Isoxazole-Based CK1 Inhibitors Modified with Chiral Pyrrolidine Scaffolds. Molecules 24.
- Schehr M, Ianes C, Weisner J, Heintze L, Muller MP, Pichlo C, Charl J, Brunstein E, Ewert J, Lehr M, Baumann U, Rauh D, Knippschild U, Peifer C, Herges R. 2019. 2-Azo-, 2-diazocine-thiazols and 2-azo-imidazoles as photoswitchable kinase inhibitors: limitations and pitfalls of the photoswitchable inhibitor approach. Photochem Photobiol Sci 18:1398-1407.
- Dolde C, Bischof J, Grüter S, Montada A, Halekotte J, Peifer C, Kalbacher H, Baumann U, Knippschild U, Suter B. 2018. A CK1 FRET biosensor reveals that DDX3X is an essential activator of CK1ε. J Cell Sci 131.Garcia-Reyes B, Witt L, Jansen B, Karasu E, Gehring T, Leban J, Henne-Bruns D, Pichlo C, Brunstein E, Baumann U, Wesseler F, Rathmer B, Schade D, Peifer C, Knippschild U. 2018. Discovery of Inhibitor of Wnt Production 2 (IWP-2) and Related Compounds As Selective ATP-Competitive Inhibitors of Casein Kinase 1 (CK1) delta/epsilon. J Med Chem 61:4087-4102.
- Halekotte J, Witt L, Ianes C, Krüger M, Buhrmann M, Rauh D, Pichlo C, Brunstein E, Luxenburger A, Baumann U, Knippschild U, Bischof J, Peifer C. 2017. Optimized 4,5-Diarylimidazoles as Potent/Selective Inhibitors of Protein Kinase CK1delta and Their Structural Relation to p38alpha MAPK. Molecules 22.
- Krüger M, Kalbacher H, Kastritis PL, Bischof J, Barth H, Henne-Bruns D, Vorgias C, Sarno S, Pinna LA, Knippschild U. 2016. New potential peptide therapeutics perturbing CK1delta/alpha-tubulin interaction. Cancer Lett 375:375-383.
- Richter J, Bischof J, Zaja M, Kohlhof H, Othersen O, Vitt D, Alscher V, Pospiech I, Garcia-Reyes B, Berg S, Leban J, Knippschild U. 2014. Difluoro-dioxolo-benzoimidazol-benzamides as potent inhibitors of CK1delta and epsilon with nanomolar inhibitory activity on cancer cell proliferation. J Med Chem 57:7933-46.
- Bischof J, Leban J, Zaja M, Grothey A, Radunsky B, Othersen O, Strobl S, Vitt D, Knippschild U. 2012. 2-Benzamido-N-(1H-benzo[d]imidazol-2-yl)thiazole-4-carboxamide derivatives as potent inhibitors of CK1delta/epsilon. Amino Acids 43:1577-91.
- Peifer C, Abadleh M, Bischof J, Hauser D, Schattel V, Hirner H, Knippschild U, Laufer S. 2009. 3,4-Diaryl-isoxazoles and -imidazoles as potent dual inhibitors of p38alpha mitogen activated protein kinase and casein kinase 1delta. J Med Chem 52:7618-30.
In brains of patients suffering from Alzheimer’s disease, increased levels of aggregated tau and amyloid-beta protein can be observed. In the underlying processes, modifications of these proteins by phosphorylation play a crucial role, e.g. by favoring the production of amyloid-beta or the formation of tau aggregates as paired helical filaments and neurofibrillary tangles, respectively. Although small molecule inhibitors could be used to efficiently inhibit kinase activity of CK1 isoforms, which are among others responsible for phosphorylation of the pathogenesis-associated proteins tau and APP (amyloid precursor protein), this approach is not satisfactory since general inhibition of CK1 isoforms might disturb other essential cellular processes also in healthy cells and tissues.
Therefore, our novel therapeutic approach does not aim on inhibition of the activity of CK1 isoforms δ and ε in general, but will block their interactions with specific cellular proteins. This is made possible by using short protein fragments, so called peptides. Instead of the enzymes CK1δ or ε these peptides will be binding to tau or APP in order to inhibit their disease-associated modification. In the following, the development of characteristic cell damage associated with neurodegenerative processes can be prevented or reduced.
The potency of therapeutic peptide candidates is first investigated by biochemical analyses before being tested in more complex cell culture models, which closely mimic the environment of a human brain affected by Alzheimer’s disease (“Alzheimer’s-in-a-dish”). Finally, the efficacy of selected therapeutic peptides is confirmed by using an appropriate animal model. Within this project potent novel therapeutic tools will be generated that can be used to effectively intervene with pathogenic processes associated with Alzheimer’s disease. These could represent an innovative starting point for the development of future approaches for the treatment of Alzheimer’s disease.
CK1-derived peptides as novel tools for the treatment of Alzheimer’s disease
Using specific peptides derived from the sequences of CK1δ and ε, the binding of these enzymes to certain substrates can be prevented. By blocking the interaction with and phosphorylation of the Alzheimer’s-associated proteins tau and APP, subsequent pathogenic processes can be inhibited resulting in prevention or reduction of neurodegeneration.
- Roth, A., Gärtner, F., Mayer, K., Beyrle, J., König, I., Knippschild, U. und Bischof, J. CK1δ-derived peptides as novel tools inhibiting the interactions between CK1δ and APP695 to modulate the pathogenic metabolism of APP. Int J Mol Sci 2021, in press
- Krüger, M., Kalbacher, H., Kastritis, P.L., Bischof, J., Barth, H., Henne-Bruns, D., Vorgias, C., Sarno, S., Pinna, L.A. und Knippschild, U. New potential peptide therapeutics perturbing CK1δ/α-tubulin interaction. Cancer Lett 2016, 375(2):375-383.
- Thal, D.R., Del Tredici, K., Ludolph, A.C., Hoozemans J.J., Rozemuller A.J., Braak H. und Knippschild U. Stages of granulovacuolar degeneration: their relation to Alzheimer's disease and chronic stress response. Acta Neuropathol 2011, 122(5):577-589.
- Bischof, J., Müller, A., Fänder, M., Knippschild, U. und Fischer, D. Neurite outgrowth of mature retinal ganglion cells and PC12 cells requires activity of CK1δ and CK1ε. PLoS One 2011, 6(6):e20857.
Key areas of our obesity-releated research:
Obesity is becoming an increasing threat to public health with a predicted prevalence of 20 % or more in European countries in 2025 and simultaneously reducing the life expectancy in OECD countries by about 3 years until 2050. One of the biggest issues is the massive growth of morbid obesity (BMI ≥ 40 kg/m2) which is tending to be at the same rate compared to milder forms. A BMI between 40 to 59 kg/m2 is associated with a loss of 6.5 to 13.7 years of life due to increased risk for diabetes, coronary heart disease, dyslipidemia, hypertension, obstructive sleep apnea, gallbladder disease, osteoarthritis and some cancers. On a molecular level, obesity is associated with a state of low-grade chronic inflammation caused by an altered pro-inflammatory secretion profile of adipose tissue that also affects the status of peripheral immune cells. For patients suffering from morbid obesity, bariatric surgical procedures often demonstrate the last opportunity to lose weight when conventional methods like diet or physical activities fail. However, the effect of the massive weight loss on the immune system needs to be further elucidated. Therefore, we have set up prospective, exploratory randomized study aims with patients undergoing a bariatric surgery in our clinic to identify factors (cytokines, hormones, adiponectin, pre-existing conditions, cell types, microbiome) that may predict the success of surgery in terms of BMI reduction (excess weight loss %). A distinction will be made between factors that approach basal levels due to weight loss and factors that remain unaffected by weight loss. Our study builds on the previous study "Establishment of a tissue and database for the molecular and biochemical characterization of blood, subcutaneous and visceral adipose tissue and liver tissue of obese patients" and "Molecular biological and biochemical characterization of pathophysiological changes in obesity and characterization of their significance for obesity-associated comorbidities". While the focus in the two studies mentioned above was on the identification of obesity-relevant factors and the characterization of the significance of obesity for the development of colorectal carcinomas, the focus is now on the identification of predictive factors that are essential for the course of obesity.
- Pradas-Juni M, Hansmeier NR, Link JC, Schmidt E, Larsen BD, Klemm P, Meola N, Topel H, Loureiro R, Dhaouadi I, Kiefer CA, Schwarzer R, Khani S, Oliverio M, Awazawa M, Frommolt P, Heeren J, Scheja L, Heine M, Dieterich C, Buning H, Yang L, Cao H, Jesus DF, Kulkarni RN, Zevnik B, Troder SE, Knippschild U, Edwards PA, Lee RG, Yamamoto M, Ulitsky I, Fernandez-Rebollo E, Vallim TQA, Kornfeld JW. 2020. A MAFG-lncRNA axis links systemic nutrient abundance to hepatic glucose metabolism. Nat Commun 11:644.
- Giroud M, Pisani DF, Karbiener M, Barquissau V, Ghandour RA, Tews D, Fischer-Posovszky P, Chambard JC, Knippschild U, Niemi T, Taittonen M, Nuutila P, Wabitsch M, Herzig S, Virtanen KA, Langin D, Scheideler M, Amri EZ. 2016. miR-125b affects mitochondrial biogenesis and impairs brite adipocyte formation and function. Mol Metab 5:615-625.
- Hillenbrand A, Kiebler B, Schwab C, Scheja L, Xu P, Henne-Bruns D, Wolf AM, Knippschild U. 2015. Prevalence of non-alcoholic fatty liver disease in four different weight related patient groups: association with small bowel length and risk factors. BMC Res Notes 8:290.
- Xu P, Fischer-Posovszky P, Bischof J, Radermacher P, Wabitsch M, Henne-Bruns D, Wolf AM, Hillenbrand A, Knippschild U. 2015. Gene expression levels of Casein kinase 1 (CK1) isoforms are correlated to adiponectin levels in adipose tissue of morbid obese patients and site-specific phosphorylation mediated by CK1 influences multimerization of adiponectin. Mol Cell Endocrinol 406:87-101.
- Shin AC, Fasshauer M, Filatova N, Grundell LA, Zielinski E, Zhou JY, Scherer T, Lindtner C, White PJ, Lapworth AL, Ilkayeva O, Knippschild U, Wolf AM, Scheja L, Grove KL, Smith RD, Qian WJ, Lynch CJ, Newgard CB, Buettner C. 2014. Brain insulin lowers circulating BCAA levels by inducing hepatic BCAA catabolism. Cell Metab 20:898-909.
- Eissing L, Scherer T, Todter K, Knippschild U, Greve JW, Buurman WA, Pinnschmidt HO, Rensen SS, Wolf AM, Bartelt A, Heeren J, Buettner C, Scheja L. 2013. De novo lipogenesis in human fat and liver is linked to ChREBP-beta and metabolic health. Nat Commun 4:1528.
- Kornfeld JW, Baitzel C, Konner AC, Nicholls HT, Vogt MC, Herrmanns K, Scheja L, Haumaitre C, Wolf AM, Knippschild U, Seibler J, Cereghini S, Heeren J, Stoffel M, Bruning JC. 2013. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature 494:111-5.
- Hillenbrand A, Fassler J, Huber N, Xu P, Henne-Bruns D, Templin M, Schrezenmeier H, Wolf AM, Knippschild U. 2012. Changed adipocytokine concentrations in colorectal tumor patients and morbidly obese patients compared to healthy controls. BMC Cancer 12:545.
- Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Bluher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. 2008. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 28:1304-10.
Worldwide, obesity and injury have become major health burdens on society. Although interactions between obesity and injury have been reported, their nature and extent is still not explored in depth. Deregulated adipose tissue functions in obesity lead to changes in released cytokines, chemokines, adipocytokines, fatty acids and hormones by adipocytes as well as resident or infiltrating macrophages finally resulting in an obesity associated chronic inflammation. Additionally, it leads to an accumulation of ectopic lipids in several organs and tissues, finally contributing to obesity related comorbidities such as heart disease, diabetes, metabolic syndrome, and a higher mortality rate due to COVID-19. Using an animal model of diet-induced obesity for tissue regeneration either after induction of blunt traumas in the muscle or in the lung, or after a combined blunt trauma in lung and muscle we could detect a delayed regeneration process in each case in obese compared to normal weight mice due to systemic changes, an altered secretion profile and differences in the response to trauma.
The state of chronic inflammation in obese individuals suggests that immune cells of obese individuals are already primed to respond to an inflammatory stimulus such as a trauma in a different manner compared to normal weight individuals. Preliminary data of our combined lung and muscle trauma model supports this hypothesis showing a prolonged presence of blood neutrophils in obese mice during the posttraumatic immune response. Additionally, an increased ratio of pro-/anti-inflammatory monocytes can be observed in the blood of obese mice during early time points post trauma. We therefore hypothesize that macrophages and infiltrating monocytes in obese individuals will deteriorate the inflammation in the muscle and the lung subsequently delaying the regeneration process.
To address this hypothesis, we will investigate the involvement of various immune cell subsets in regeneration and response after trauma and thereby focusing on the influence of obesity to changes in the immune reaction after induction of a combined muscle and lung trauma. Finally, the in vivo findings in mice will be translated to the clinical setting with further analyses of immune cell deregulations in obese and normal weight patients of a human polytrauma cohort.
- Gihring A, Gärtner F, Liu C, Hoenicka M, Wabitsch M, Knippschild U, Xu P. 2020. Influence of Obesity on the Organization of the Extracellular Matrix and Satellite Cell Functions After Combined Muscle and Thorax Trauma in C57BL/6J Mice. Front Physiol 11:849.
- Xu P, Gärtner F, Gihring A, Liu C, Burster T, Wabitsch M, Knippschild U, Paschke S. 2020. Influence of obesity on remodeling of lung tissue and organization of extracellular matrix after blunt thorax trauma. Respir Res 21:238.
- Werner JU, Todter K, Xu P, Lockhart L, Jahnert M, Gottmann P, Schurmann A, Scheja L, Wabitsch M, Knippschild U. 2018. Comparison of Fatty Acid and Gene Profiles in Skeletal Muscle in Normal and Obese C57BL/6J Mice before and after Blunt Muscle Injury. Front Physiol 9:19.
- Xu P, Werner JU, Milerski S, Hamp CM, Kuzenko T, Jahnert M, Gottmann P, de Roy L, Warnecke D, Abaei A, Palmer A, Huber-Lang M, Durselen L, Rasche V, Schürmann A, Wabitsch M, Knippschild U. 2018. Diet-Induced Obesity Affects Muscle Regeneration After Murine Blunt Muscle Trauma-A Broad Spectrum Analysis. Front Physiol 9:674.
Head of the working group
PD Dr. rer. nat. Joachim Bischof (2013-7/2021)
Dr. med. Pengfei Xu (2011-12/2020)
2023: Aileen Roth, Adrian Gihring
2021: M. Sc. Fabian Gärtner (estimated nov/dec)
2019: Dr. rer. nat. Chiara Ianes
2018: Dr. rer. nat. Jens-Uwe Werner
2017: Dr. biol. hum. Balbina Garcia Reyes
2016: Dr. rer. nat. Marc Krüger
2015: Dr. rer. nat. Julia Richter
2013: Dr. rer. nat. Joachim Andreas Bischof
2011: Dr. biol. hum. Kalim Ullah Khan
2010: Dr. rer. nat. Heidrun Hirner
2006: Dr. biol. hum. Georgios Giamas
2004: Dr. rer. nat. Sonja Alexandra Wolff
2000: Dr. rer. nat. Lars Behrend
2020: Dr. med. Congxing Liu
2018: Dr. med. Sina Christine Friederike Freimooser
2015: Dr. med. Sebastian Berg, Dr. med. Zhigang Meng
2014: Dr. med. Elena Felicia Wurster
2013: Dr. med. Sebastian Berg, Dr. Sven Jannis Randoll
2012: Dr. med. Stefan Keifenheim, Dr. med. Franziska Oltmer, Dr. med. Barbara Radunsky
2011: Dr. med. Pengfei Xu
2009: Dr. Holger Scharneck, Dr. med. Anja Christine Utz
2007: Dr. med. Levani Shoshiashvili
2006: Dr. med. Nadine Huber
2004: Dr. med. Claas Brockschmidt
2022: MSc Linda Grabe
2021: MSc Johann Weber, MSc Florian Göser, MSc Laura Meier
2020: MSc Dominik Pflumm
2019: MSc Adrian Gihring, MSc Aileen Roth
2018: MSc Lucia Gallo, MSc Andrea Gilg, MSc Philipp Haas, MSc Sonja Katharina Schmidt
2017: MSc Carmen Hamp, MSc Ebru-Kubra Karasu, MSc Tanja Kuzenko
2016: MSc Elif Eminowski, MSc Lydia Lockhart, MSc Anna-Laura Kretz, MSc Thomas Raphael Böhm
2015: MSc Chiara Ianes, MSc Ivana Sfarcic
2014: MSc Natalie Werz, MSc Nan Zhang
2013: MSc Marc Krüger
2009: MSc Joachim Bischof
2002: Dipl. Biochem. Tanja Maritzen, Dipl. Biol. Matthias Piesche
2000: Dipl. Biochem. Martin Stöter
2022: Lorenz Marte, Laura Mayer
2021: Vincent Ohlhauser, Marleen Oswald, Anabelle Sander
2020: Julian Beyrle, Linda Grabe, Laura Starcke, Irina König, Melissa Lock
2019: Katja Mayer
2018: Toni Schadler
2017: Marie-Therese Bammert, Vanessa Hardt, Jonas Peter Kammer
2016: Tanja Gehring, Julius Trübenbach
2015: Lefin Maunick Koloko Ngassie
2013: Sina Bühler, Mario Müller, Sebastian Pilsl
2011: Raphael Lattke
2008: Simone Bammert, Sonja Kern,
2007: Joachim Bischof, Anil Demirata, Corinna Patzina
2022
Tian X, Traub B, Shi J, Huber N, Schreiner S, Chen G, Zhou S, Henne-Bruns D, Knippschild U, Kornmann M. c-Jun N-terminal kinase 2 suppresses pancreatic cancer growth and invasion and is opposed by c-Jun N-terminal kinase 1. Cancer Gene Ther. 2022 Jan;29(1):73-86. doi: 10.1038/s41417-020-00290-5. Epub 2021 Feb 1. PMID: 33526844; PMCID: PMC8761571. IF: 5.854
Smirlis D, Dingli F, Sabatet V, Roth A, Knippschild U, Loew D, Späth GF, Rachidi N. Identification of the Host Substratome of Leishmania-Secreted Casein Kinase 1 Using a SILAC-Based Quantitative Mass Spectrometry Assay. Front Cell Dev Biol. 2022 Jan 3;9:800098. doi: 10.3389/fcell.2021.800098. PMID: 35047509; PMCID: PMC8762337. IF: 6,081
von Drathen T, Ure EM, Kirschner S, Roth A, Meier L, Woolhouse AD, Cameron SA, Knippschild U, Peifer C, Luxenburger A. C5-Iminosugar modification of casein kinase 1δ lead 3-(4-fluorophenyl)-5-isopropyl-4-(pyridin-4-yl)isoxazole promotes enhanced inhibitor affinity and selectivity. Arch Pharm (Weinheim). 2022 May;355(5):e2100497. doi: 10.1002/ardp.202100497. Epub 2022 Feb 17. PMID: 35174898. IF: 4,631
Xu P, Westhoff MA, Hadzalic A, Debatin KM, Winiarski L, Oleksyszyn J, Wirtz CR, Knippschild U, Burster T. Diisothiocyanate-Derived Mercapturic Acids Are a Promising Partner for Combination Therapies in Glioblastoma. ACS Omega. 2022 Feb 8;7(7):5929-5936. doi: 10.1021/acsomega.1c06169. PMID: 35224353; PMCID: PMC8867792. IF: 4,132
Xing H, Zhang Y, Krämer M, Kissmann AK, Amann V, Raber HF, Weil T, Stieger KR, Knippschild U, Henkel M, Andersson J, Rosenau F. A Polyclonal Aptamer Library for the Specific Binding of the Gut Bacterium Roseburia intestinalis in Mixtures with Other Gut Microbiome Bacteria and Human Stool Samples. Int J Mol Sci. 2022 Jul 13;23(14):7744. doi: 10.3390/ijms23147744. PMID: 35887092; PMCID: PMC9317077. IF: 6,208
Xing H, Zhang Y, Krämer M, Kissmann AK, Henkel M, Weil T, Knippschild U, Rosenau F. A Polyclonal Selex Aptamer Library Directly Allows Specific Labelling of the Human Gut Bacterium Blautia producta without Isolating IndividualAptamers. Molecules. 2022 Sep 3;27(17):5693. doi: 10.3390/molecules27175693. PMID: 36080459; PMCID: PMC9458011. IF: 4,927
Roth A, Sander A, Oswald MS, Gärtner F, Knippschild U, Bischof J. Comprehensive Characterization of CK1δ-Mediated Tau Phosphorylation in Alzheimer's Disease. Front Mol Biosci. 2022 Jun 27;9:872171. doi: 10.3389/fmolb.2022.872171. PMID: 36203870; PMCID: PMC9531328. IF: 6,133
Wesseler F, Lohmann S, Riege D, Halver J, Roth A, Pichlo C, Weber S, Takamiya M, Müller E, Ketzel J, Flegel J, Gihring A, Rastegar S, Bertrand J, Baumann U, Knippschild U, Peifer C, Sievers S, Waldmann H, Schade D. Phenotypic Discovery of Triazolo[1,5-c]quinazolines as a First-In-Class Bone Morphogenetic Protein Amplifier Chemotype. J Med Chem. 2022 Nov 8. doi: 10.1021/acs.jmedchem.2c01199. Epub ahead of print. PMID: 36346705. IF: 8,093
2022
El Hage R, Knippschild U, Arnold T, Hinterseher I. Stem Cell-Based Therapy: A Promising Treatment for Diabetic Foot Ulcer. Biomedicines. 2022 Jun 25;10(7):1507. doi: 10.3390/biomedicines10071507. PMID: 35884812; PMCID: PMC9312797. IF: 4,757
Bagci-Onder T, Kutuk O, Chonghaile TN, Knippschild U. Editorial: Cell Death and Targeted Cancer Therapies. Front Cell Dev Biol. 2022 Jul 6;10:967720. doi: 10.3389/fcell.2022.967720. PMID: 35874828; PMCID: PMC9296767. IF: 6,081
Gihring A, Gärtner F, Schirmer M, Wabitsch M, Knippschild U. Recent Developments in Mouse Trauma Research Models: A Mini-Review. Front Physiol. 2022 Apr 29;13:866617. doi: 10.3389/fphys.2022.866617. PMID: 35574493; PMCID: PMC9101050. IF: 4,755
Roth A, Gihring A, Bischof J, Pan L, Oswald F, Knippschild U. CK1 Is a Druggable Regulator of Microtubule Dynamics and Microtubule-Associated Processes. Cancers (Basel). 2022 Mar 5;14(5):1345. doi: 10.3390/cancers14051345. PMID: 35267653; PMCID: PMC8909099. IF: 6,575
2021
Smirlis D, Dingli F, Sabatet V, Roth A, Knippschild U, Loew D, Spaeth G, Rachidi N. 2021. Identification of the host substratome of Leishmania-secreted casein kinase 1 using a SILAC-based quantitative mass spectrometry assay. Frontiers in Cell and Developmental Biology. epub
Gärtner F, Gihring A, Roth A, Bischof J, Xu P, Elad L, Wabitsch M, Burster T, Uwe Knippschild U. 2021. Obesity prolongs the inflammatory response in mice after severe trauma and attenuates the splenic response to the inflammatory reflex. Front Immunol. epub
Burster T, Gartner F, Bulach C, Zhanapiya A, Gihring A, Knippschild U. 2021. Regulation of MHC I Molecules in Glioblastoma Cells and the Sensitizing of NK Cells. Pharmaceuticals (Basel) 14(3) https://www.ncbi.nlm.nih.gov/pubmed/33800301. 10.3390/ph14030236
Burster T, Gartner F, Knippschild U, Zhanapiya A. 2021. Activity-Based Probes to Utilize the Proteolytic Activity of Cathepsin G in Biological Samples. Front Chem 9: p. 628295 https://www.ncbi.nlm.nih.gov/pubmed/33732686. 10.3389/fchem.2021.628295
Burster T, Traut R, Yermekkyzy Z, Mayer K, Westhoff M A, Bischof J, Knippschild U. 2021. Critical View of Novel Treatment Strategies for Glioblastoma: Failure and Success of Resistance Mechanisms by Glioblastoma Cells. Front Cell Dev Biol 9: p. 695325 https://www.ncbi.nlm.nih.gov/pubmed/34485282. 10.3389/fcell.2021.695325
Pan L, Hoffmeister P, Turkiewicz A, Huynh N N D, Grosse-Berkenbusch A, Knippschild U, Gebhardt J C M, Baumann B, Borggrefe T, Oswald F. 2021. Transcription Factor RBPJL Is Able to Repress Notch Target Gene Expression but Is Non-Responsive to Notch Activation. Cancers (Basel) 13(19) https://www.ncbi.nlm.nih.gov/pubmed/34638511. 10.3390/cancers13195027
Raber H F, Kubiczek D H, Bodenberger N, Kissmann A K, D'Souza D, Xing H, Mayer D, Xu P, Knippschild U, Spellerberg B, Weil T, Rosenau F. 2021. FluCell-SELEX Aptamers as Specific Binding Molecules for Diagnostics of the Health Relevant Gut Bacterium Akkermansia muciniphila. Int J Mol Sci 22(19) https://www.ncbi.nlm.nih.gov/pubmed/34638764. 10.3390/ijms221910425
Rachidi N, Knippschild U, Spath G F. 2021. Dangerous Duplicity: The Dual Functions of Casein Kinase 1 in Parasite Biology and Host Subversion. Front Cell Infect Microbiol 11: p. 655700 https://www.ncbi.nlm.nih.gov/pubmed/33869086. 10.3389/fcimb.2021.655700
Roth A, Gartner F, Mayer K, Beyrle J, Konig I, Knippschild U, Bischof J. 2021. CK1delta-Derived Peptides as Novel Tools Inhibiting the Interactions between CK1delta and APP695 to Modulate the Pathogenic Metabolism of APP. Int J Mol Sci 22(12) https://www.ncbi.nlm.nih.gov/pubmed/34203978. 10.3390/ijms22126423
Roth A, Gihring A, Goser F, Peifer C, Knippschild U, Bischof J. 2021. Assessing the Inhibitory Potential of Kinase Inhibitors In Vitro: Major Pitfalls and Suggestions for Improving Comparability of Data Using CK1 Inhibitors as an Example. Molecules 26(16) https://www.ncbi.nlm.nih.gov/pubmed/34443486. 10.3390/molecules26164898
Tian X, Traub B, Shi J, Huber N, Schreiner S, Chen G, Zhou S, Henne-Bruns D, Knippschild U, Kornmann M. 2021. c-Jun N-terminal kinase 2 suppresses pancreatic cancer growth and invasion and is opposed by c-Jun N-terminal kinase 1. Cancer Gene Ther https://www.ncbi.nlm.nih.gov/pubmed/33526844. 10.1038/s41417-020-00290-5
Traub B, Roth A, Kornmann M, Knippschild U, Bischof J. 2021. Stress-activated kinases as therapeutic targets in pancreatic cancer. World J Gastroenterol 27(30): p. 4963-4984 https://www.ncbi.nlm.nih.gov/pubmed/34497429. 10.3748/wjg.v27.i30.4963
2020
Xu P, Richter J, Blatz A, Gartner F, Alberts R, Azoitei A, Makori WA, Meessen S, Knippschild U, Gunes C. 2020. Downregulation of ORP3 Correlates with Reduced Survival of Colon Cancer Patients with Advanced Nodal Metastasis and of Female Patients with Grade 3 Colon Cancer. Int J Mol Sci 21. pubmed.ncbi.nlm.nih.gov/32824360/
Tian X, Traub B, Xie X, Zhou S, Henne-Bruns D, Knippschild U, Kornmann M. 2020. Opposing Oncogenic Functions of p38 Mitogen-activated Protein Kinase Alpha and Beta in Human Pancreatic Cancer Cells. Anticancer Res 40:5545-5556. pubmed.ncbi.nlm.nih.gov/32988878/
Njeru SN, Kraus J, Meena JK, Lechel A, Katz SF, Kumar M, Knippschild U, Azoitei A, Wezel F, Bolenz C, Leithauser F, Gollowitzer A, Omrani O, Hoischen C, Koeberle A, Kestler HA, Gunes C, Rudolph KL. 2020. Aneuploidy-inducing gene knockdowns overlap with cancer mutations and identify Orp3 as a B-cell lymphoma suppressor. Oncogene 39:1445-1465. pubmed.ncbi.nlm.nih.gov/31659255/
Gärtner F, Knippschild U, Burster T. 2020. Application of an Activity-Based Probe to Determine Proteolytic Activity of Cell Surface Cathepsin G by Mass Cytometry Data Acquisition. ACS Omega 5:28233-28238. pubmed.ncbi.nlm.nih.gov/33163806/
Burster T, Knippschild U, Molnar F, Zhanapiya A. 2020. Cathepsin G and its Dichotomous Role in Modulating Levels of MHC Class I Molecules. Arch Immunol Ther Exp (Warsz) 68:25. pubmed.ncbi.nlm.nih.gov/32815043/
Xu P, Gärtner F, Gihring A, Liu C, Burster T, Wabitsch M, Knippschild U, Paschke S. 2020. Influence of obesity on remodeling of lung tissue and organization of extracellular matrix after blunt thorax trauma. Respir Res 21:238. pubmed.ncbi.nlm.nih.gov/32943048/
Gihring A, Gärtner F, Liu C, Hoenicka M, Wabitsch M, Knippschild U, Xu P. 2020. Influence of Obesity on the Organization of the Extracellular Matrix and Satellite Cell Functions After Combined Muscle and Thorax Trauma in C57BL/6J Mice. Front Physiol 11:849. pubmed.ncbi.nlm.nih.gov/32848828/
Pradas-Juni M, Hansmeier NR, Link JC, Schmidt E, Larsen BD, Klemm P, Meola N, Topel H, Loureiro R, Dhaouadi I, Kiefer CA, Schwarzer R, Khani S, Oliverio M, Awazawa M, Frommolt P, Heeren J, Scheja L, Heine M, Dieterich C, Buning H, Yang L, Cao H, Jesus DF, Kulkarni RN, Zevnik B, Troder SE, Knippschild U, Edwards PA, Lee RG, Yamamoto M, Ulitsky I, Fernandez-Rebollo E, Vallim TQA, Kornfeld JW. 2020. A MAFG-lncRNA axis links systemic nutrient abundance to hepatic glucose metabolism. Nat Commun 11:644. pubmed.ncbi.nlm.nih.gov/32005828/
2019
Xu P, Ianes C, Gärtner F, Liu C, Burster T, Bakulev V, Rachidi N, Knippschild U, Bischof J. 2019. Structure, regulation, and (patho-)physiological functions of the stress-induced protein kinase CK1 delta (CSNK1D). Gene 715:144005. pubmed.ncbi.nlm.nih.gov/31376410/
Schehr M, Ianes C, Weisner J, Heintze L, Muller MP, Pichlo C, Charl J, Brunstein E, Ewert J, Lehr M, Baumann U, Rauh D, Knippschild U, Peifer C, Herges R. 2019. 2-Azo-, 2-diazocine-thiazols and 2-azo-imidazoles as photoswitchable kinase inhibitors: limitations and pitfalls of the photoswitchable inhibitor approach. Photochem Photobiol Sci 18:1398-1407. pubmed.ncbi.nlm.nih.gov/30924488/
Meng Z, Bohm T, Xu P, Henne-Bruns D, Peifer C, Witt L, Knippschild U, Bischof J. 2019. Kinase activity of casein kinase 1 delta (CK1delta) is modulated by protein kinase C alpha (PKCalpha) by site-specific phosphorylation within the kinase domain of CK1delta. Biochim Biophys Acta Proteins Proteom 1867:710-721. pubmed.ncbi.nlm.nih.gov/31096047/
Luxenburger A, Schmidt D, Ianes C, Pichlo C, Kruger M, von Drathen T, Brunstein E, Gainsford GJ, Baumann U, Knippschild U, Peifer C. 2019. Design, Synthesis and Biological Evaluation of Isoxazole-Based CK1 Inhibitors Modified with Chiral Pyrrolidine Scaffolds. Molecules 24. pubmed.ncbi.nlm.nih.gov/30832206/
Liu C, Witt L, Ianes C, Bischof J, Bammert MT, Baier J, Kirschner S, Henne-Bruns D, Xu P, Kornmann M, Peifer C, Knippschild U. 2019. Newly Developed CK1-Specific Inhibitors Show Specifically Stronger Effects on CK1 Mutants and Colon Cancer Cell Lines. Int J Mol Sci 20. pubmed.ncbi.nlm.nih.gov/31817920/
Giamas G, Hirner H, Shoshiashvili L, Grothey A, Gessert S, Kuhl M, Henne-Bruns D, Vorgias CE, Knippschild U. 2019. Correction: Phosphorylation of CK1delta: identification of Ser(370) as the major phosphorylation site targeted by PKA in vitro and in vivo. Biochem J 476:1221-1225. pubmed.ncbi.nlm.nih.gov/31000625/
Bohm T, Meng Z, Haas P, Henne-Bruns D, Rachidi N, Knippschild U, Bischof J. 2019. The kinase domain of CK1delta can be phosphorylated by Chk1. Biosci Biotechnol Biochem 83:1663-1675. pubmed.ncbi.nlm.nih.gov/31094292/
Bischof J, Gartner F, Zeiser K, Kunz R, Schreiner C, Hoffer E, Burster T, Knippschild U, Zimecki M. 2019. Immune Cells and Immunosenescence. Folia Biol (Praha) 65:53-63. pubmed.ncbi.nlm.nih.gov/31464181/
Kretz AL, Trauzold A, Hillenbrand A, Knippschild U, Henne-Bruns D, von Karstedt S, Lemke J. 2019. TRAILblazing Strategies for Cancer Treatment. Cancers (Basel) 11. pubmed.ncbi.nlm.nih.gov/30935038/
Dimitrakopoulos C, Vrugt B, Flury R, Schraml P, Knippschild U, Wild P, Hoerstrup S, Henne-Bruns D, Wuerl P, Graf R, Breitenstein S, Bond G, Beerenwinkel N, Grochola LF. 2019. Identification and Validation of a Biomarker Signature in Patients With Resectable Pancreatic Cancer via Genome-Wide Screening for Functional Genetic Variants. JAMA Surg 154:e190484. pubmed.ncbi.nlm.nih.gov/30942874/
Liu Y, Zhang L, Li W, Huang Q, Yuan S, Li Y, Liu J, Zhang S, Pin G, Song S, Ray PF, Arnoult C, Cho C, Garcia-Reyes B, Knippschild U, Strauss JF, Zhang Z. 2019. The sperm-associated antigen 6 interactome and its role in spermatogenesis. Reproduction 158:181-197.
2018
Richter J, Kretz AL, Lemke J, Fauler M, Werner JU, Paschke S, Leithauser F, Henne-Bruns D, Hillenbrand A, Knippschild U. 2018. CK1alpha overexpression correlates with poor survival in colorectal cancer. BMC Cancer 18:140. pubmed.ncbi.nlm.nih.gov/29409464/
Garcia-Reyes B, Witt L, Jansen B, Karasu E, Gehring T, Leban J, Henne-Bruns D, Pichlo C, Brunstein E, Baumann U, Wesseler F, Rathmer B, Schade D, Peifer C, Knippschild U. 2018. Discovery of Inhibitor of Wnt Production 2 (IWP-2) and Related Compounds As Selective ATP-Competitive Inhibitors of Casein Kinase 1 (CK1) delta/epsilon. J Med Chem 61:4087-4102. pubmed.ncbi.nlm.nih.gov/29630366/
Garcia-Reyes B, Kretz AL, Ruff JP, von Karstedt S, Hillenbrand A, Knippschild U, Henne-Bruns D, Lemke J. 2018. The Emerging Role of Cyclin-Dependent Kinases (CDKs) in Pancreatic Ductal Adenocarcinoma. Int J Mol Sci 19. pubmed.ncbi.nlm.nih.gov/30340359/
Dolde C, Bischof J, Gruter S, Montada A, Halekotte J, Peifer C, Kalbacher H, Baumann U, Knippschild U, Suter B. 2018. A CK1 FRET biosensor reveals that DDX3X is an essential activator of CK1epsilon. J Cell Sci 131. pubmed.ncbi.nlm.nih.gov/29222110/
Bischof J, Kretz AL, Burster T, Henne-Bruns D, Knippschild U, Xu P. 2018. Inhibition of CK1 affects Viability and Survival of Glioblastoma Cells. Biomed J Sci & Tech Res 2. biomedres.us/pdfs/BJSTR.MS.ID.000735.pdf
Cwiklowska K, Westhoff MA, Freisinger S, Dwucet A, Halatsch ME, Knippschild U, Debatin KM, Schirmbeck R, Winiarski L, Oleksyszyn J, Wirtz CR, Burster T. 2018. Viability of glioblastoma stem cells is effectively reduced by diisothiocyanate-derived mercapturic acids. Oncol Lett 16:6181-6187. pubmed.ncbi.nlm.nih.gov/30344758/
Kretz AL, von Karstedt S, Hillenbrand A, Henne-Bruns D, Knippschild U, Trauzold A, Lemke J. 2018. Should We Keep Walking along the Trail for Pancreatic Cancer Treatment? Revisiting TNF-Related Apoptosis-Inducing Ligand for Anticancer Therapy. Cancers (Basel) 10. https://pubmed.ncbi.nlm.nih.gov/29562636/
Werner JU, Tödter K, Xu P, Lockhart L, Jahnert M, Gottmann P, Schurmann A, Scheja L, Wabitsch M, Knippschild U. 2018. Comparison of Fatty Acid and Gene Profiles in Skeletal Muscle in Normal and Obese C57BL/6J Mice before and after Blunt Muscle Injury. Front Physiol 9:19. pubmed.ncbi.nlm.nih.gov/29441023/
Xu P, Werner JU, Milerski S, Hamp CM, Kuzenko T, Jahnert M, Gottmann P, de Roy L, Warnecke D, Abaei A, Palmer A, Huber-Lang M, Dürselen L, Rasche V, Schurmann A, Wabitsch M, Knippschild U. 2018. Diet-Induced Obesity Affects Muscle Regeneration After Murine Blunt Muscle Trauma-A Broad Spectrum Analysis. Front Physiol 9:674. pubmed.ncbi.nlm.nih.gov/29922174/
Wille C, Eiseler T, Langenberger ST, Richter J, Mizuno K, Radermacher P, Knippschild U, Huber-Lang M, Seufferlein T, Paschke S. 2018. PKD regulates actin polymerization, neutrophil deformability, and transendothelial migration in response to fMLP and trauma. J Leukoc Biol 104:615-630. pubmed.ncbi.nlm.nih.gov/29656400/
2017
Wolff S, Xiao Z, Wittau M, Sussner N, Stöter M, Knippschild U. 2017. Corrigendum to "interaction of casein kinase 1 delta (CK1d) with the light chain LC2 of microtubule associated protein 1A (MAP1A)" [Biochim. Biophys. Acta 1745/2 (2005) 196-2006]. Biochim Biophys Acta Mol Cell Res 1864:1923. pubmed.ncbi.nlm.nih.gov/28571942/
Halekotte J, Witt L, Ianes C, Krüger M, Buhrmann M, Rauh D, Pichlo C, Brunstein E, Luxenburger A, Baumann U, Knippschild U, Bischof J, Peifer C. 2017. Optimized 4,5-Diarylimidazoles as Potent/Selective Inhibitors of Protein Kinase CK1delta and Their Structural Relation to p38alpha MAPK. Molecules 22. pubmed.ncbi.nlm.nih.gov/28338621/
Föhr KJ, Knippschild U, Herkommer A, Fauler M, Peifer C, Georgieff M, Adolph O. 2017. State-dependent block of voltage-gated sodium channels by the casein-kinase 1 inhibitor IC261. Invest New Drugs 35:277-289. pubmed.ncbi.nlm.nih.gov/28164251/
Bischof J, Westhoff MA, Wagner JE, Halatsch ME, Trentmann S, Knippschild U, Wirtz CR, Burster T. 2017. Cancer stem cells: The potential role of autophagy, proteolysis, and cathepsins in glioblastoma stem cells. Tumour Biol 39:1010428317692227. pubmed.ncbi.nlm.nih.gov/28347245/
Kretz AL, Schaum M, Richter J, Kitzig EF, Engler CC, Leithauser F, Henne-Bruns D, Knippschild U, Lemke J. 2017. CDK9 is a prognostic marker and therapeutic target in pancreatic cancer. Tumour Biol 39:1010428317694304. pubmed.ncbi.nlm.nih.gov/28231737/
Traub B, Sun L, Ma Y, Xu P, Lemke J, Paschke S, Henne-Bruns D, Knippschild U, Kornmann M. 2017. Endogenously Expressed IL-4Ralpha Promotes the Malignant Phenotype of Human Pancreatic Cancer In Vitro and In Vivo. Int J Mol Sci 18. pubmed.ncbi.nlm.nih.gov/28350325/
Rupakova N, Bakulev V, Knippschild U, Garcia-Reyes B, Eltsov O, Slesarev P, Beliaev N, Slepukhin P, Witt L, Peifer C, Beryozkina T. 2017. Design and synthesis of N-benzimidazol-2-yl-N'-sulfonyl acetamidines. Archive for Organic Chemistry:15. www.arkat-usa.org/get-file/60765/
2016
Richter J, Rudeck S, Kretz AL, Kramer K, Just S, Henne-Bruns D, Hillenbrand A, Leithauser F, Lemke J, Knippschild U. 2016. Decreased CK1delta expression predicts prolonged survival in colorectal cancer patients. Tumour Biol 37:8731-9. pubmed.ncbi.nlm.nih.gov/26738869/
Meng Z, Bischof J, Ianes C, Henne-Bruns D, Xu P, Knippschild U. 2016. CK1delta kinase activity is modulated by protein kinase C alpha (PKCalpha)-mediated site-specific phosphorylation. Amino Acids 48:1185-97. pubmed.ncbi.nlm.nih.gov/26803658/
Krüger M, Kalbacher H, Kastritis PL, Bischof J, Barth H, Henne-Bruns D, Vorgias C, Sarno S, Pinna LA, Knippschild U. 2016. New potential peptide therapeutics perturbing CK1delta/alpha-tubulin interaction. Cancer Lett 375:375-383. pubmed.ncbi.nlm.nih.gov/26996302/
Ianes C, Xu P, Werz N, Meng Z, Henne-Bruns D, Bischof J, Knippschild U. 2016. CK1delta activity is modulated by CDK2/E- and CDK5/p35-mediated phosphorylation. Amino Acids 48:579-92. pubmed.ncbi.nlm.nih.gov/26464264/
Hillenbrand A, Xu P, Zhou S, Blatz A, Weiss M, Hafner S, Henne-Bruns D, Knippschild U. 2016. Circulating adipokine levels and prognostic value in septic patients. J Inflamm (Lond) 13:30. pubmed.ncbi.nlm.nih.gov/27601939/
Giroud M, Pisani DF, Karbiener M, Barquissau V, Ghandour RA, Tews D, Fischer-Posovszky P, Chambard JC, Knippschild U, Niemi T, Taittonen M, Nuutila P, Wabitsch M, Herzig S, Virtanen KA, Langin D, Scheideler M, Amri EZ. 2016. miR-125b affects mitochondrial biogenesis and impairs brite adipocyte formation and function. Mol Metab 5:615-625. pubmed.ncbi.nlm.nih.gov/27656399/
Yokoyama S, Higashi M, Kitamoto S, Oeldorf M, Knippschild U, Kornmann M, Maemura K, Kurahara H, Wiest E, Hamada T, Kitazono I, Goto Y, Tasaki T, Hiraki T, Hatanaka K, Mataki Y, Taguchi H, Hashimoto S, Batra SK, Tanimoto A, Yonezawa S, Hollingsworth MA. 2016. Aberrant methylation of MUC1 and MUC4 promoters are potential prognostic biomarkers for pancreatic ductal adenocarcinomas. Oncotarget 7:42553-42565. pubmed.ncbi.nlm.nih.gov/27283771/
Udelnow A, Henne-Bruns D, Knippschild U, Halangk W, Bruns C, Halloul Z, Wurl P, Grochola LF. 2016. MDM2 SNP 309G Allele Is Associated With Younger Age at Surgery in Chronic Pancreatitis Patients. Pancreas 45:e11-2. pubmed.ncbi.nlm.nih.gov/26954495/
Stracquadanio G, Vrugt B, Flury R, Schraml P, Würl P, Muller TH, Knippschild U, Henne-Bruns D, Breitenstein S, Clavien PA, Graf R, Bond GL, Grochola LF. 2016. CD44 SNPrs187115: A Novel Biomarker Signature that Predicts Survival in Resectable Pancreatic Ductal Adenocarcinoma. Clin Cancer Res 22:6069-6077. pubmed.ncbi.nlm.nih.gov/27283965/
Schmieder M, Henne-Bruns D, Mayer B, Knippschild U, Rolke C, Schwab M, Kramer K. 2016. Comparison of Different Risk Classification Systems in 558 Patients with Gastrointestinal Stromal Tumors after R0-Resection. Front Pharmacol 7:504. pubmed.ncbi.nlm.nih.gov/28082898/
2015
Wolff S, Garcia-Reyes B, Henne-Bruns D, Bischof J, Knippschild U. 2015. Protein kinase CK1 interacts with and phosphorylates RanBPM in vitro. Journal of Molecular Biochemistry 4:8.
Winkler BS, Oltmer F, Richter J, Bischof J, Xu P, Burster T, Leithauser F, Knippschild U. 2015. CK1delta in lymphoma: gene expression and mutation analyses and validation of CK1delta kinase activity for therapeutic application. Front Cell Dev Biol 3:9. pubmed.ncbi.nlm.nih.gov/25750912/
Richter J, Ullah K, Xu P, Alscher V, Blatz A, Peifer C, Halekotte J, Leban J, Vitt D, Holzmann K, Bakulev V, Pinna LA, Henne-Bruns D, Hillenbrand A, Kornmann M, Leithauser F, Bischof J, Knippschild U. 2015. Effects of altered expression and activity levels of CK1delta and varepsilon on tumor growth and survival of colorectal cancer patients. Int J Cancer 136:2799-810. pubmed.ncbi.nlm.nih.gov/25404202/
Lott S, Schmieder M, Mayer B, Henne-Bruns D, Knippschild U, Agaimy A, Schwab M, Kramer K. 2015. Gastrointestinal stromal tumors of the esophagus: evaluation of a pooled case series regarding clinicopathological features and clinical outcome. Am J Cancer Res 5:333-43. pubmed.ncbi.nlm.nih.gov/25628942/
Kramer K, Wolf S, Mayer B, Schmidt SA, Agaimy A, Henne-Bruns D, Knippschild U, Schwab M, Schmieder M. 2015. Frequence, spectrum and prognostic impact of additional malignancies in patients with gastrointestinal stromal tumors. Neoplasia 17:134-40. pubmed.ncbi.nlm.nih.gov/25622906/
Kramer K, Knippschild U, Mayer B, Bogelspacher K, Spatz H, Henne-Bruns D, Agaimy A, Schwab M, Schmieder M. 2015. Impact of age and gender on tumor related prognosis in gastrointestinal stromal tumors (GIST). BMC Cancer 15:57. pubmed.ncbi.nlm.nih.gov/25886494/
Hillenbrand A, Kiebler B, Schwab C, Scheja L, Xu P, Henne-Bruns D, Wolf AM, Knippschild U. 2015. Prevalence of non-alcoholic fatty liver disease in four different weight related patient groups: association with small bowel length and risk factors. BMC Res Notes 8:290. pubmed.ncbi.nlm.nih.gov/26138508/
Xu P, Fischer-Posovszky P, Bischof J, Radermacher P, Wabitsch M, Henne-Bruns D, Wolf AM, Hillenbrand A, Knippschild U. 2015. Gene expression levels of Casein kinase 1 (CK1) isoforms are correlated to adiponectin levels in adipose tissue of morbid obese patients and site-specific phosphorylation mediated by CK1 influences multimerization of adiponectin. Mol Cell Endocrinol 406:87-101. pubmed.ncbi.nlm.nih.gov/25724478/
Rozin Y, Zhidovinov S, Beryozkina T, Shafran Y, Lubec G, Eltsov O, Slepukhin P, Knippschild U, Bischof J, Dehaen W, Bakulev V. 2015. A novel transformation of beta-1,2,3-thiadiazol-5-yl enamines into thieno[3,3-d]pyridazines. Tetrahedron Letters 56:3. www.sciencedirect.com/science/article/abs/pii/S0040403915002725
2014
Stöter M, Kruger M, Banting G, Henne-Bruns D, Knippschild U. 2014. Microtubules depolymerization caused by the CK1 inhibitor IC261 may be not mediated by CK1 blockage. PLoS One 9:e100090. pubmed.ncbi.nlm.nih.gov/24937750/
Richter J, Bischof J, Zaja M, Kohlhof H, Othersen O, Vitt D, Alscher V, Pospiech I, Garcia-Reyes B, Berg S, Leban J, Knippschild U. 2014. Difluoro-dioxolo-benzoimidazol-benzamides as potent inhibitors of CK1delta and epsilon with nanomolar inhibitory activity on cancer cell proliferation. J Med Chem 57:7933-46. pubmed.ncbi.nlm.nih.gov/25191940/
Knippschild U, Kruger M, Richter J, Xu P, Garcia-Reyes B, Peifer C, Halekotte J, Bakulev V, Bischof J. 2014. The CK1 Family: Contribution to Cellular Stress Response and Its Role in Carcinogenesis. Front Oncol 4:96. pubmed.ncbi.nlm.nih.gov/24904820/
Haselmann V, Kurz A, Bertsch U, Hubner S, Olempska-Muller M, Fritsch J, Hasler R, Pickl A, Fritsche H, Annewanter F, Engler C, Fleig B, Bernt A, Roder C, Schmidt H, Gelhaus C, Hauser C, Egberts JH, Heneweer C, Rohde AM, Boger C, Knippschild U, Rocken C, Adam D, Walczak H, Schutze S, Janssen O, Wulczyn FG, Wajant H, Kalthoff H, Trauzold A. 2014. Nuclear death receptor TRAIL-R2 inhibits maturation of let-7 and promotes proliferation of pancreatic and other tumor cells. Gastroenterology 146:278-90. pubmed.ncbi.nlm.nih.gov/24120475/
Radermacher P, Huber-Lang M, Knippschild U, Thiemermann C. 2014. The obesity paradox revisited: does increased body mass protect against circulatory shock? Shock 41:554-5. pubmed.ncbi.nlm.nih.gov/24830951/
Shin AC, Fasshauer M, Filatova N, Grundell LA, Zielinski E, Zhou JY, Scherer T, Lindtner C, White PJ, Lapworth AL, Ilkayeva O, Knippschild U, Wolf AM, Scheja L, Grove KL, Smith RD, Qian WJ, Lynch CJ, Newgard CB, Buettner C. 2014. Brain insulin lowers circulating BCAA levels by inducing hepatic BCAA catabolism. Cell Metab 20:898-909. pubmed.ncbi.nlm.nih.gov/25307860/
Efimov I, Bakulev V, Beliaev N, Beryozkina T, Knippschild U, Leban J, Zhi-Jin F, Eltsov O, Slepukhin P, Ezhikova M, Dehaen W. 2014. Reactions of β‐Azolylenamines with Sulfonyl Azides as an Approach to N‐Unsubstituted 1,2,3‐Triazoles and Ethene‐1,2‐diamines. European Journal of Organic Chemistry doi:10.1002/ejoc.201402130:5. chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/ejoc.201402130
2013
Bischof J, Randoll SJ, Süssner N, Henne-Bruns D, Pinna LA, Knippschild U. 2013. CK1delta kinase activity is modulated by Chk1-mediated phosphorylation. PLoS One 8:e68803. pubmed.ncbi.nlm.nih.gov/23861943/
Wang JC, Thiere M, Henne-Bruns D, Knippschild U, Kornmann M. 2013. Inhibition of pancreatic cancer cell growth in vivo using a tetracycline-inducible cyclin D1 antisense expression system. Pancreas 42:141-8. pubmed.ncbi.nlm.nih.gov/22722256/
Kumar M, Witt B, Knippschild U, Koch S, Meena JK, Heinlein C, Weise JM, Krepulat F, Kuchenbauer F, Iben S, Rudolph KL, Deppert W, Gunes C. 2013. CEBP factors regulate telomerase reverse transcriptase promoter activity in whey acidic protein-T mice during mammary carcinogenesis. Int J Cancer 132:2032-43. pubmed.ncbi.nlm.nih.gov/23023397/
Kornfeld JW, Baitzel C, Konner AC, Nicholls HT, Vogt MC, Herrmanns K, Scheja L, Haumaitre C, Wolf AM, Knippschild U, Seibler J, Cereghini S, Heeren J, Stoffel M, Bruning JC. 2013. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature 494:111-5. pubmed.ncbi.nlm.nih.gov/23389544/
Hafner S, Hillenbrand A, Knippschild U, Radermacher P. 2013. The obesity paradox and acute kidney injury: beneficial effects of hyper-inflammation? Crit Care 17:1023. pubmed.ncbi.nlm.nih.gov/24326122/
Eissing L, Scherer T, Todter K, Knippschild U, Greve JW, Buurman WA, Pinnschmidt HO, Rensen SS, Wolf AM, Bartelt A, Heeren J, Buettner C, Scheja L. 2013. De novo lipogenesis in human fat and liver is linked to ChREBP-beta and metabolic health. Nat Commun 4:1528. pubmed.ncbi.nlm.nih.gov/23443556/
2012
Hirner H, Gunes C, Bischof J, Wolff S, Grothey A, Kuhl M, Oswald F, Wegwitz F, Bosl MR, Trauzold A, Henne-Bruns D, Peifer C, Leithauser F, Deppert W, Knippschild U. 2012. Impaired CK1 delta activity attenuates SV40-induced cellular transformation in vitro and mouse mammary carcinogenesis in vivo. PLoS One 7:e29709. pubmed.ncbi.nlm.nih.gov/22235331/
Bischof J, Leban J, Zaja M, Grothey A, Radunsky B, Othersen O, Strobl S, Vitt D, Knippschild U. 2012. 2-Benzamido-N-(1H-benzo[d]imidazol-2-yl)thiazole-4-carboxamide derivatives as potent inhibitors of CK1delta/epsilon. Amino Acids 43:1577-91. pubmed.ncbi.nlm.nih.gov/22331384/
Knippschild U, Peifer C, Bischof J, Kramer K. 2012. Other tyorisne kinase inhibitors in development and future directions, Tyrosine kinase inhibitors for gastrointestinal stromal tumors, Future Medicine. www.futuremedicine.com/doi/book/10.2217/9781780840505
Hillenbrand A, Weiss M, Knippschild U, Wolf AM, Huber-Lang M. 2012. Sepsis-Induced Adipokine Change with regard to Insulin Resistance. Int J Inflam 2012:972368. pubmed.ncbi.nlm.nih.gov/22272381/
Hillenbrand A, Fassler J, Huber N, Xu P, Henne-Bruns D, Templin M, Schrezenmeier H, Wolf AM, Knippschild U. 2012. Changed adipocytokine concentrations in colorectal tumor patients and morbidly obese patients compared to healthy controls. BMC Cancer 12:545. pubmed.ncbi.nlm.nih.gov/23173608/
2011
Bischof J, Müller A, Fander M, Knippschild U, Fischer D. 2011. Neurite outgrowth of mature retinal ganglion cells and PC12 cells requires activity of CK1delta and CK1epsilon. PLoS One 6:e20857. pubmed.ncbi.nlm.nih.gov/21698236/
Thal DR, Del Tredici K, Ludolph AC, Hoozemans JJ, Rozemuller AJ, Braak H, Knippschild U. 2011. Stages of granulovacuolar degeneration: their relation to Alzheimer's disease and chronic stress response. Acta Neuropathol 122:577-89. pubmed.ncbi.nlm.nih.gov/21935637/
Udelnow A, Kreyes A, Ellinger S, Landfester K, Walther P, Klapperstueck T, Wohlrab J, Henne-Bruns D, Knippschild U, Würl P. 2011. Omeprazole inhibits proliferation and modulates autophagy in pancreatic cancer cells. PLoS One 6:e20143. pubmed.ncbi.nlm.nih.gov/21629657/
Wacker SA, Alvarado C, von Wichert G, Knippschild U, Wiedenmann J, Clauss K, Nienhaus GU, Hameister H, Baumann B, Borggrefe T, Knöchel W, Oswald F. 2011. RITA, a novel modulator of Notch signalling, acts via nuclear export of RBP-J. EMBO J 30:43-56. pubmed.ncbi.nlm.nih.gov/21102556/
Hillenbrand A, Weiss M, Knippschild U, Stromeyer HG, Henne-Bruns D, Huber-Lang M, Wolf AM. 2011. Association of adiponectin levels and insulin demand in critically ill patients. Diabetes Metab Syndr Obes 4:45-51. pubmed.ncbi.nlm.nih.gov/21448321/
2010
Utz AC, Hirner H, Blatz A, Hillenbrand A, Schmidt B, Deppert W, Henne-Bruns D, Fischer D, Thal DR, Leithauser F, Knippschild U. 2010. Analysis of cell type-specific expression of CK1 epsilon in various tissues of young adult BALB/c Mice and in mammary tumors of SV40 T-Ag-transgenic mice. J Histochem Cytochem 58:1-15. pubmed.ncbi.nlm.nih.gov/19755715/
Giamas G, Man YL, Hirner H, Bischof J, Kramer K, Khan K, Ahmed SS, Stebbing J, Knippschild U. 2010. Kinases as targets in the treatment of solid tumors. Cell Signal 22:984-1002. pubmed.ncbi.nlm.nih.gov/20096351/
Dorn J, Spatz H, Schmieder M, Barth TF, Blatz A, Henne-Bruns D, Knippschild U, Kramer K. 2010. Cyclin H expression is increased in GIST with very-high risk of malignancy. BMC Cancer 10:350. pubmed.ncbi.nlm.nih.gov/20598140/
Hauk TG, Leibinger M, Muller A, Andreadaki A, Knippschild U, Fischer D. 2010. Stimulation of axon regeneration in the mature optic nerve by intravitreal application of the toll-like receptor 2 agonist Pam3Cys. Invest Ophthalmol Vis Sci 51:459-64. pubmed.ncbi.nlm.nih.gov/19661221/
Hillenbrand A, Knippschild U, Weiss M, Schrezenmeier H, Henne-Bruns D, Huber-Lang M, Wolf AM. 2010. Sepsis induced changes of adipokines and cytokines - septic patients compared to morbidly obese patients. BMC Surg 10:26. pubmed.ncbi.nlm.nih.gov/20825686/
2009
Peifer C, Abadleh M, Bischof J, Hauser D, Schattel V, Hirner H, Knippschild U, Laufer S. 2009. 3,4-Diaryl-isoxazoles and -imidazoles as potent dual inhibitors of p38alpha mitogen activated protein kinase and casein kinase 1delta. J Med Chem 52:7618-30. pubmed.ncbi.nlm.nih.gov/19591487/
Löhler J, Hirner H, Schmidt B, Kramer K, Fischer D, Thal DR, Leithauser F, Knippschild U. 2009. Immunohistochemical characterisation of cell-type specific expression of CK1delta in various tissues of young adult BALB/c mice. PLoS One 4:e4174. pubmed.ncbi.nlm.nih.gov/19137063/
2008
Brockschmidt C, Hirner H, Huber N, Eismann T, Hillenbrand A, Giamas G, Radunsky B, Ammerpohl O, Bohm B, Henne-Bruns D, Kalthoff H, Leithauser F, Trauzold A, Knippschild U. 2008. Anti-apoptotic and growth-stimulatory functions of CK1 delta and epsilon in ductal adenocarcinoma of the pancreas are inhibited by IC261 in vitro and in vivo. Gut 57:799-806. pubmed.ncbi.nlm.nih.gov/18203806/
Schmieder M, Wolf S, Danner B, Stoehr S, Juchems MS, Wuerl P, Henne-Bruns D, Knippschild U, Hasel C, Kramer K. 2008. p16 expression differentiates high-risk gastrointestinal stromal tumor and predicts poor outcome. Neoplasia 10:1154-62. pubmed.ncbi.nlm.nih.gov/18813351/
Grochola LF, Greither T, Taubert HW, Moller P, Knippschild U, Udelnow A, Henne-Bruns D, Wurl P. 2008. Prognostic relevance of hTERT mRNA expression in ductal adenocarcinoma of the pancreas. Neoplasia 10:973-6. pubmed.ncbi.nlm.nih.gov/18714398/
Grochola LF, Greither T, Taubert H, Moller P, Knippschild U, Udelnow A, Henne-Bruns D, Wurl P. 2008. The stem cell-associated Hiwi gene in human adenocarcinoma of the pancreas: expression and risk of tumour-related death. Br J Cancer 99:1083-8. pubmed.ncbi.nlm.nih.gov/18781170/
Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Bluher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. 2008. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 28:1304-10. pubmed.ncbi.nlm.nih.gov/18420999/
2007
Giamas G, Hirner H, Shoshiashvili L, Grothey A, Gessert S, Kuhl M, Henne-Bruns D, Vorgias CE, Knippschild U. 2007. Phosphorylation of CK1delta: identification of Ser370 as the major phosphorylation site targeted by PKA in vitro and in vivo. Biochem J 406:389-98. pubmed.ncbi.nlm.nih.gov/17594292/
von Blume J, Knippschild U, Dequiedt F, Giamas G, Beck A, Auer A, Van Lint J, Adler G, Seufferlein T. 2007. Phosphorylation at Ser244 by CK1 determines nuclear localization and substrate targeting of PKD2. EMBO J 26:4619-33. pubmed.ncbi.nlm.nih.gov/17962809/
Giamas G, Stebbing J, Vorgias CE, Knippschild U. 2007. Protein kinases as targets for cancer treatment. Pharmacogenomics 8:1005-16. pubmed.ncbi.nlm.nih.gov/17716234/
2006
Wolff S, Stoter M, Giamas G, Piesche M, Henne-Bruns D, Banting G, Knippschild U. 2006. Casein kinase 1 delta (CK1delta) interacts with the SNARE associated protein snapin. FEBS Lett 580:6477-84. pubmed.ncbi.nlm.nih.gov/17101137/
2005
Wolff S, Xiao Z, Wittau M, Süssner N, Stöter M, Knippschild U. 2005. Interaction of casein kinase 1 delta (CK1 delta) with the light chain LC2 of microtubule associated protein 1A (MAP1A). Biochim Biophys Acta 1745:196-206. pubmed.ncbi.nlm.nih.gov/15961172/
Stöter M, Bamberger AM, Aslan B, Kurth M, Speidel D, Loning T, Frank HG, Kaufmann P, Lohler J, Henne-Bruns D, Deppert W, Knippschild U. 2005. Inhibition of casein kinase I delta alters mitotic spindle formation and induces apoptosis in trophoblast cells. Oncogene 24:7964-75. pubmed.ncbi.nlm.nih.gov/16027726/
Knippschild U, Wolff S, Giamas G, Brockschmidt C, Wittau M, Würl PU, Eismann T, Stöter M. 2005. The role of the casein kinase 1 (CK1) family in different signaling pathways linked to cancer development. Onkologie 28:508-14. pubmed.ncbi.nlm.nih.gov/16186692/
Knippschild U, Gocht A, Wolff S, Huber N, Lohler J, Stöter M. 2005. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal 17:675-89. pubmed.ncbi.nlm.nih.gov/15722192/
2004
Winter M, Milne D, Dias S, Kulikov R, Knippschild U, Blattner C, Meek D. 2004. Protein kinase CK1delta phosphorylates key sites in the acidic domain of murine double-minute clone 2 protein (MDM2) that regulate p53 turnover. Biochemistry 43:16356-64. pubmed.ncbi.nlm.nih.gov/15610030/
Ventura RA, Martin-Subero JI, Knippschild U, Gascoyne RD, Delsol G, Mason DY, Siebert R. 2004. Centrosome abnormalities in ALK-positive anaplastic large-cell lymphoma. Leukemia 18:1910-1. pubmed.ncbi.nlm.nih.gov/15385940/
2003
Meek DW, Knippschild U. 2003. Posttranslational modification of MDM2. Mol Cancer Res 1:1017-26. pubmed.ncbi.nlm.nih.gov/14707285/
Martin-Subero JI, Knippschild U, Harder L, Barth TF, Riemke J, Grohmann S, Gesk S, Hoppner J, Moller P, Parwaresch RM, Siebert R. 2003. Segmental chromosomal aberrations and centrosome amplifications: pathogenetic mechanisms in Hodgkin and Reed-Sternberg cells of classical Hodgkin's lymphoma? Leukemia 17:2214-9. pubmed.ncbi.nlm.nih.gov/14523479/
Maritzen T, Löhler J, Deppert W, Knippschild U. 2003. Casein kinase I delta (CKIdelta) is involved in lymphocyte physiology. Eur J Cell Biol 82:369-78. pubmed.ncbi.nlm.nih.gov/12924632/
2000
Behrend L, Stöter M, Kurth M, Rutter G, Heukeshoven J, Deppert W, Knippschild U. 2000. Interaction of casein kinase 1 delta (CK1delta) with post-Golgi structures, microtubules and the spindle apparatus. Eur J Cell Biol 79:240-51. pubmed.ncbi.nlm.nih.gov/10826492/
Behrend L, Milne DM, Stöter M, Deppert W, Campbell LE, Meek DW, Knippschild U. 2000. IC261, a specific inhibitor of the protein kinases casein kinase 1-delta and -epsilon, triggers the mitotic checkpoint and induces p53-dependent postmitotic effects. Oncogene 19:5303-13. pubmed.ncbi.nlm.nih.gov/11103931/
1999
Janus F, Albrechtsen N, Knippschild U, Wiesmüller L, Grosse F, Deppert W. 1999. Different regulation of the p53 core domain activities 3'-to-5' exonuclease and sequence-specific DNA binding. Mol Cell Biol 19:2155-68. pubmed.ncbi.nlm.nih.gov/10022902/
1997
Meek DW, Campbell LE, Jardine LJ, Knippschild U, McKendrick L, Milne DM. 1997. Multi-site phosphorylation of p53 by protein kinases inducible by p53 and DNA damage. Biochem Soc Trans 25:416-9.
Knippschild U, Milne DM, Campbell LE, DeMaggio AJ, Christenson E, Hoekstra MF, Meek DW. 1997. p53 is phosphorylated in vitro and in vivo by the delta and epsilon isoforms of casein kinase 1 and enhances the level of casein kinase 1 delta in response to topoisomerase-directed drugs. Oncogene 15:1727-36. pubmed.ncbi.nlm.nih.gov/9349507/
Henning W, Rohaly G, Kolzau T, Knippschild U, Maacke H, Deppert W. 1997. MDM2 is a target of simian virus 40 in cellular transformation and during lytic infection. J Virol 71:7609-18. pubmed.ncbi.nlm.nih.gov/9311842/
1996
Knippschild U, Oren M, Deppert W. 1996. Abrogation of wild-type p53 mediated growth-inhibition by nuclear exclusion. Oncogene 12:1755-65. pubmed.ncbi.nlm.nih.gov/9191128/
Knippschild U, Milne D, Campbell L, Meek D. 1996. p53 N-terminus-targeted protein kinase activity is stimulated in response to wild type p53 and DNA damage. Oncogene 13:1387-93. pubmed.ncbi.nlm.nih.gov/8875976/
Stürzbecher HW, Donzelmann B, Henning W, Knippschild U, Buchhop S. 1996. p53 is linked directly to homologous recombination processes via RAD51/RecA protein interaction. EMBO J 15:1992-2002. pubmed.ncbi.nlm.nih.gov/8617246/
1995
Knippschild U, Kolzau T, Deppert W. 1995. Cell-specific transcriptional activation of the mdm2-gene by ectopically expressed wild-type form of a temperature-sensitive mutant p53. Oncogene 11:683-90. pubmed.ncbi.nlm.nih.gov/7651732/
1993
Zerrahn J, Knippschild U, Winkler T, Deppert W. 1993. Independent expression of the transforming amino-terminal domain of SV40 large I antigen from an alternatively spliced third SV40 early mRNA. EMBO J 12:4739-46. pubmed.ncbi.nlm.nih.gov/8223482/
1992
Patschinsky T, Knippschild U, Deppert W. 1992. Species-specific phosphorylation of mouse and rat p53 in simian virus 40-transformed cells. J Virol 66:3846-59. pubmed.ncbi.nlm.nih.gov/1316485/
1991
Knippschild U, Kiefer J, Patschinsky T, Deppert W. 1991. Phenotype-specific phosphorylation of simian virus 40 tsA mutant large T antigens in tsA N-type and A-type transformants. J Virol 65:4414-23. pubmed.ncbi.nlm.nih.gov/1649337/
Deppert W, Kurth M, Graessmann M, Graessmann A, Knippschild U. 1991. Altered phosphorylation at specific sites confers a mutant phenotype to SV40 wild-type large T antigen in a flat revertant of SV40-transformed cells. Oncogene 6:1931-8. pubmed.ncbi.nlm.nih.gov/1923516/