Entwicklungs- und Tumorimmunologie (AG Jumaa)
Antibody diversity is achieved by random recombination of immunoglobulin (Ig) gene segments in developing B lymphocyte precursors. While this process is essential to yield highly diverse B cell antigen receptors (BCRs), it bears the risk of producing receptors with specificities that may recognize and lead to the destruction of self-structures. Traditional thoughts have mainly focused on how such putatively dangerous BCR specificities are dealt with and how they contribute to the development of autoimmune diseases. In contrast to this traditional view, our work has established a positive and even necessary role for self-recognition during early B cell development. According to our model, the importance of auto-reactivity led to the development of a specialized auto-reactive precursor BCR (pre-BCR), whose main function is to provide developing cells with the auto-reactivity required for selection. As these results provide the basis for a novel concept for the generation of antibody-secreting cells, our future work will focus on how self-structures are actually protected from the action of the own immune system. We are using a murine IL7-based in vitro culture experimental system that allows the propagation of unlimited numbers of primary precursor B cells and we have developed this system for reconstitution studies that aim at investigating the molecular mechanisms for the proliferation, differentiation and transformation of early B cells. By combining this system with transgenic mice, we were able to identify and characterize the in vivo function of crucial elements for the development of B cells.
The BCR is a central regulator of B lymphocyte differentiation and proliferation. We are interested in how signalling from BCRs may promote lymphoma development and whether there are structural and/or functional features specific for BCRs expressed on lymphoma cells. Based on our previous studies on autonomous signalling of BCRs from polyreactive B cells, we have characterized BCRs from patients with Chronic Lymphocytic Leukemia (CLL). We could show that, in contrast to other B cell neoplasias, such as Multiple Myeloma, Mantle Cell Myeloma, Marginal Zone Myeloma and Follicular Lymphoma, CLL-derived BCRs induce antigen-independent, cell-autonomous signalling which is dependent on the heavy chain complementarity determining region 3 (HCDR3) and an internal epitope in framework region 2 of the BCR heavy chain (HC). Transferring the HCDR3 of CLL-derived BCRs confers autonomous signalling capacity to non-autonomously active BCRs, whereas mutations in the internal epitope abolish this capacity. In this ongoing project, we further characterize structural and functional features of BCRs from lymphoma patients and are in the process of identifying bio-molecules suitable to specifically influence signalling from autonomously active receptors.
Since B cells can express different BCR isotypes depending on the class of the receptor heavy chain, we address the question whether BCR isotypes play differential roles in B cell activation and whether this is relevant for the pathogenesis human B cell malignancies.
The non-receptor protein tyrosine kinase Syk is a key mediator of signal transduction in B cells. By acting downstream of the BCR, Syk promotes signalling pathways involved in proliferation, differentiation and in the survival of transformed B cells. The goal of this project is to analyze the mechanisms and kinetics of Syk-induced proliferation and malignant transformation. To this end, we generated a mouse model for the inducible expression of the leukemia-derived TEL-Syk (TS) fusion protein using a tamoxifen-inducible Cre mouse line for B cell-specific expression of TEL-Syk in adult mice. This study shows that TS expression leads to a marked transient expansion of the B cell pool in the periphery. However, our results suggest that inducible expression of TS in B cells is not sufficient for the transformation of B cells, as corresponding cells react with escape mechanisms leading to expression of tumor suppressors and initiation of terminal differentiation that limit the survival and expansion of the activated B cell. In a related project, we analyze the effects of expression of ITK-Syk, another leukemia-derived Syk fusion protein.
The Phosphatidylinositol-3-Kinase (PI3-K) pathway is an important signalling axis in B-lymphocytes. It is activated after BCR engagement and regulates the activity of important downstream targets including the forkhead box class O (FoxO) transcription factors, which are well-known tumour suppressors. FoxO proteins require Pten(phosphatase and tensin homologue, which counteracts PI3-K activity) for activation and play important roles in the regulation of B cell development and function. However, an important question is how a ubiquitously expressed gene such as FoxO1 can activate tissue-specific processes. We have recently shown that Pten and the downstream factor FoxO1 are required for the expression of Ikaros, a transcription factor required for lymphocyte development and tumour suppression. For instance, more than 80% of human BCR/Abl-positive B-ALL cases have mutations or deletions in IKZF1, the gene encoding Ikaros, and mutations in IKZF1 in other sub-types of B-ALL correlate with poor prognosis. Interestingly, our results suggest that splicing of Ikaros pre-mRNA is disturbed in the absence of Pten or FoxO1. This leads to the production of an alternative transcript that apparently does not encode any Ikaros protein. We want to investigate the molecular mechanisms of posttranscriptional regulation of Ikaros expression and aim to identify the involved factors.To this end, we are performing genome wide high-throughput RNA sequencing (RNA-Seq) experiments in Pten-deficient as compared with reconstituted cells and have identified potentially involved splicing factors. Moreover, we plan to investigate the expression of the aberrant Ikaros transcripts and the identified splicing factors in human ALL samples.
The role of Pten/FoxO1/Ikaros in later stages of B cell development is not yet characterized and we assume that this axis is important to regulate the proliferation and function of activated mature B cells. For instance, lack of Pten has been shown to lead to elevated numbers of B cells and splenomegaly in the respective animals. These Pten-deficient cells are resistant to the induction of apoptosis suggesting that the accumulation of these B cells is induced by prolonged survival. We want to investigate the expression of Ikaros as well as the role of the genes identified in the RNA-Seq experiments in the proliferation of Pten-deficient B cells. With these experiments, we plan to establish the Pten/FoxO1/Ikaros as a tumour suppressor axis that regulates the expansion of B cells. in fact, PI3K-inhibitors seem to be effective in the treatment of human B cell malignancies suggesting that the Pten-mediated processes are somehow blocked in these cells. Therefore, characterizing the Pten/FoxO1/Ikaros axis and identification of downstream targets might help to improve the treatment of malignancies.
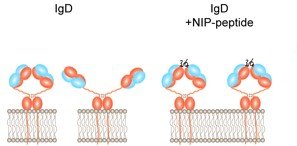
In our recent study published by Nature Immunology, we investigated the requirements and the physiological consequences of the activation of the B cell receptor (BCR) isotypes IgM and IgD. Although evolutionary conserved and tightly regulated, the role of IgD in B cell development is not understood and the dominant IgD expression in mature B cells is unclear.
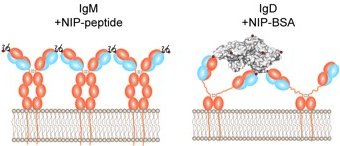
Our study shows that IgD requires complex antigen for stimulation while monovalent antigens cannot activate IgD. In fact, our study even shows that monovalent antigen prevents the activation of mature IgD-expressing B cells by complex antigen, thereby identifying an unexpected level of regulation in which soluble antigen competes with complex antigen for BCR binding and B cell activation. Thus, the increased IgD expression during maturation shifts the responsiveness of conventional B cells towards complex antigens by upregulation of IgD expression. The underlying mechanism is provided by a specific region in IgD, called the hinge region.
A specialized B cell population designated B1 B cells, which produce natural autoantibodies, need increased BCR signaling for development and express reduced amounts of IgD as compared to IgM. B1 B cells are innate-like in the absence of previous exposure to foreign antigens. In addition to protection from pathogens, B1 B cells fulfill important housekeeping functions including the prevention of inflammation and auto-immune diseases. The reduced IgD versus IgM expression on B1 B cells seems to be important for their homeostasis and function as revealed by analysis of IgDnull and IgMnull mice.
These data not only answer the long-standing question about the difference between the IgM- and IgD-BCR, they also establish a novel concept for immune regulation and open up new opportunities for improving the vaccination approaches aimed at activation of specific B cell populations to generate antibodies that protect from from pathogens or autoimmune disorders.
This project aims at investigating the molecular mechanisms that activate B cell antigen receptor (BCR) signalling in chronic lymphocytic leukaemia (CLL). While it is widely accepted that the unbroken BCR expression in CLL cells is indicative for a key role in disease development, the mechanisms that induce BCR activation and survival of malignant cells are still elusive. Using a unique reconstitution system, we have recently shown that CLL-derived BCRs possess the exceptional capacity for cell-autonomous signalling independent of external antigen. Crystallographic analyses confirmed our model that CLL-BCRs bind to intrinsic motifs in nearby BCRs on the very same cell. In addition to the BCR, several pathogenic factors influence the biological behaviour of CLL cells, but the functional hierarchy and the effect on BCR signalling are insufficiently understood. Here, we aim at investigating the structural cause of autonomous signalling as well as the characterization of important signalling pathways and their mechanistic action in CLL pathogenesis.
By combining crystallography with the measurement of autonomous signalling of wild type and mutated receptors in an unique reconstitution system, we will generate a structure-function relationship for CLL-BCRs. By generating new animal models and by employing classical as well as cutting-edge approaches of biochemistry and molecular/cellular immunology, we will comprehensively characterize the signalling pathways that are activated by autonomous signalling and might be important for CLL pathogenesis.
These systematic efforts are necessary to understand how various biological mechanisms operate and ultimately activate downstream pathways that result in a lymphoproliferative disease. In addition, a cohesive model of CLL pathogenesis, which elucidates the hierarchical order of pathogenic factors and their interaction with BCR signalling, may well lead to novel disease-specific preventive or therapeutic intervention.
Benkisser-Petersen, M., M. Buchner, A. Dorffel, M. Duhren-von-Minden, R. Claus, K. Klasener, K. Leberecht, M. Burger, C. Dierks, H. Jumaa, F. Malavasi, M. Reth, H. Veelken, J. Duyster, and K. Zirlik. 2016. Spleen Tyrosine Kinase Is Involved in the CD38 Signal Transduction Pathway in Chronic Lymphocytic Leukemia. PLoS One 11:e0169159.
Hobeika, E., P. C. Maity, and H. Jumaa. 2016. Control of B Cell Responsiveness by Isotype and Structural Elements of the Antigen Receptor. Trends Immunol 37:310-20.
Ubelhart, R., M. Werner, and H. Jumaa. 2016. Assembly and Function of the Precursor B-Cell Receptor. Curr Top Microbiol Immunol 393:3-25.
Shojaee, S., L. N. Chan, M. Buchner, V. Cazzaniga, K. N. Cosgun, H. Geng, Y. H. Qiu, M. D. von Minden, T. Ernst, A. Hochhaus, G. Cazzaniga, A. Melnick, S. M. Kornblau, T. G. Graeber, H. Wu, H. Jumaa, and M. Muschen. 2016. PTEN opposes negative selection and enables oncogenic transformation of pre-B cells. Nat Med 22:379-87.
Kohler, M., M. Roring, B. Schorch, K. Heilmann, N. Stickel, G. J. Fiala, L. C. Schmitt, S. Braun, S. Ehrenfeld, F. M. Uhl, T. Kaltenbacher, F. Weinberg, S. Herzog, R. Zeiser, W. W. Schamel, H. Jumaa, and T. Brummer. 2016. Activation loop phosphorylation regulates B-Raf in vivo and transformation by B-Raf mutants. EMBO J 35:143-61.
Kohrer, S., O. Havranek, F. Seyfried, C. Hurtz, G. P. Coffey, E. Kim, E. Ten Hacken, U. Jager, K. Vanura, S. O'Brien, D. A. Thomas, H. Kantarjian, D. Ghosh, Z. Wang, M. Zhang, W. Ma, H. Jumaa, K. M. Debatin, M. Muschen, L. H. Meyer, R. E. Davis, and J. A. Burger. 2016. Pre-BCR signaling in precursor B-cell acute lymphoblastic leukemia regulates PI3K/AKT, FOXO1 and MYC, and can be targeted by SYK inhibition. Leukemia 30:1246-54.
Schneider, D., M. Duhren-von Minden, A. Alkhatib, C. Setz, C. A. van Bergen, M. Benkisser-Petersen, I. Wilhelm, S. Villringer, S. Krysov, G. Packham, K. Zirlik, W. Romer, C. Buske, F. K. Stevenson, H. Veelken, and H. Jumaa. 2015. Lectins from opportunistic bacteria interact with acquired variable-region glycans of surface immunoglobulin in follicular lymphoma. Blood 125:3287-96.
Flemming, A., Q. Q. Huang, J. P. Jin, H. Jumaa, and S. Herzog. 2015. A Conditional Knockout Mouse Model Reveals That Calponin-3 Is Dispensable for Early B Cell Development. PLoS One 10:e0128385.
Sen, S., M. Langiewicz, H. Jumaa, and N. J. Webster. 2015. Deletion of serine/arginine-rich splicing factor 3 in hepatocytes predisposes to hepatocellular carcinoma in mice. Hepatology 61:171-83.
Maity, P. C., A. Blount, H. Jumaa, O. Ronneberger, B. F. Lillemeier, and M. Reth. 2015. B cell antigen receptors of the IgM and IgD classes are clustered in different protein islands that are altered during B cell activation. Sci Signal 8:ra93.
Kumar, R., M. P. Bach, F. Mainoldi, M. Maruya, S. Kishigami, H. Jumaa, T. Wakayama, O. Kanagawa, S. Fagarasan, and S. Casola. 2015. Antibody repertoire diversification through VH gene replacement in mice cloned from an IgA plasma cell. Proc Natl Acad Sci U S A 112:E450-7.
Ubelhart, R., E. Hug, M. P. Bach, T. Wossning, M. Duhren-von Minden, A. H. Horn, D. Tsiantoulas, K. Kometani, T. Kurosaki, C. J. Binder, H. Sticht, L. Nitschke, M. Reth, and H. Jumaa. 2015. Responsiveness of B cells is regulated by the hinge region of IgD. Nat Immunol 16:534-43.
Ubelhart, R., and H. Jumaa. 2015. Autoreactivity and the positive selection of B cells. Eur J Immunol 45:2971-7.
Chen, Z., S. Shojaee, M. Buchner, H. Geng, J. W. Lee, L. Klemm, B. Titz, T. G. Graeber, E. Park, Y. X. Tan, A. Satterthwaite, E. Paietta, S. P. Hunger, C. L. Willman, A. Melnick, M. L. Loh, J. U. Jung, J. E. Coligan, S. Bolland, T. W. Mak, A. Limnander, H. Jumaa, M. Reth, A. Weiss, C. A. Lowell, and M. Muschen. 2015. Signalling thresholds and negative B-cell selection in acute lymphoblastic leukaemia. Nature 521:357-61.
Jumaa, H. 2015. Tuning B cell responsiveness by antigen receptor isotype. Oncotarget 6:32311-2.
Iacovelli, S., E. Hug, S. Bennardo, M. Duehren-von Minden, S. Gobessi, A. Rinaldi, M. Suljagic, D. Bilbao, G. Bolasco, J. Eckl-Dorna, V. Niederberger, F. Autore, S. Sica, L. Laurenti, H. Wang, R. J. Cornall, S. H. Clarke, C. M. Croce, F. Bertoni, H. Jumaa, and D. G. Efremov. 2015. Two types of BCR interactions are positively selected during leukemia development in the Emu-TCL1 transgenic mouse model of CLL. Blood 125:1578-88.
Hug, E., E. Hobeika, M. Reth, and H. Jumaa. 2014. Inducible expression of hyperactive Syk in B cells activates Blimp-1-dependent terminal differentiation. Oncogene 33:3730-41.
Sprissler, C., D. Belenki, H. Maurer, K. Aumann, D. Pfeifer, C. Klein, T. A. Muller, S. Kissel, J. Hulsdunker, J. Alexandrovski, T. Brummer, H. Jumaa, J. Duyster, and C. Dierks. 2014. Depletion of STAT5 blocks TEL-SYK-induced APMF-type leukemia with myelofibrosis and myelodysplasia in mice. Blood Cancer J 4:e240.
Surova, E., and H. Jumaa. 2014. The role of BCR isotype in B-cell development and activation. Adv Immunol 123:101-39.
Decker, S., J. Finter, A. J. Forde, S. Kissel, J. Schwaller, T. S. Mack, A. Kuhn, N. Gray, M. Follo, H. Jumaa, M. Burger, K. Zirlik, D. Pfeifer, C. V. Miduturu, H. Eibel, H. Veelken, and C. Dierks. 2014. PIM kinases are essential for chronic lymphocytic leukemia cell survival (PIM2/3) and CXCR4-mediated microenvironmental interactions (PIM1). Mol Cancer Ther 13:1231-45.
Bach, M. P., D. Schneider, and H. Jumaa. 2014. [Autoreactivity in B cell development]. Z Rheumatol 73:62-4.
Bach, M. P., E. Hug, M. Werner, J. Holch, C. Sprissler, K. Pechloff, K. Zirlik, R. Zeiser, C. Dierks, J. Ruland, and H. Jumaa. 2014. Premature terminal differentiation protects from deregulated lymphocyte activation by ITK-Syk. J Immunol 192:1024-33.
Sen, S., H. Jumaa, and N. J. Webster. 2013. Splicing factor SRSF3 is crucial for hepatocyte differentiation and metabolic function. Nat Commun 4:1336.
Ramezani-Rad, P., H. Geng, C. Hurtz, L. N. Chan, Z. Chen, H. Jumaa, A. Melnick, E. Paietta, W. L. Carroll, C. L. Willman, V. Lefebvre, and M. Muschen. 2013. SOX4 enables oncogenic survival signals in acute lymphoblastic leukemia. Blood 121:148-55.
Yaktapour, N., R. Ubelhart, J. Schuler, K. Aumann, C. Dierks, M. Burger, D. Pfeifer, H. Jumaa, H. Veelken, T. Brummer, and K. Zirlik. 2013. Insulin-like growth factor-1 receptor (IGF1R) as a novel target in chronic lymphocytic leukemia. Blood 122:1621-33.
Linka, R. M., S. L. Risse, K. Bienemann, M. Werner, Y. Linka, F. Krux, C. Synaeve, R. Deenen, S. Ginzel, R. Dvorsky, M. Gombert, A. Halenius, R. Hartig, M. Helminen, A. Fischer, P. Stepensky, K. Vettenranta, K. Kohrer, M. R. Ahmadian, H. J. Laws, B. Fleckenstein, H. Jumaa, S. Latour, B. Schraven, and A. Borkhardt. 2012. Loss-of-function mutations within the IL-2 inducible kinase ITK in patients with EBV-associated lymphoproliferative diseases. Leukemia 26:963-71.
Ta, V. B., M. J. de Bruijn, L. Matheson, M. Zoller, M. P. Bach, H. Wardemann, H. Jumaa, A. Corcoran, and R. W. Hendriks. 2012. Highly restricted usage of Ig H chain VH14 family gene segments in Slp65-deficient pre-B cell leukemia in mice. J Immunol 189:4842-51.
Werner, M., and H. Jumaa. 2012. DOCKing innate to adaptive signaling for persistent antibody production. Nat Immunol 13:525-6.
Alkhatib, A., M. Werner, E. Hug, S. Herzog, C. Eschbach, H. Faraidun, F. Kohler, T. Wossning, and H. Jumaa. 2012. FoxO1 induces Ikaros splicing to promote immunoglobulin gene recombination. J Exp Med 209:395-406.
Decker, S., K. Zirlik, L. Djebatchie, D. Hartmann, G. Ihorst, A. Schmitt-Graeff, D. Herchenbach, H. Jumaa, M. Warmuth, H. Veelken, and C. Dierks. 2012. Trisomy 12 and elevated GLI1 and PTCH1 transcript levels are biomarkers for Hedgehog-inhibitor responsiveness in CLL. Blood 119:997-1007.
Duhren-von Minden, M., R. Ubelhart, D. Schneider, T. Wossning, M. P. Bach, M. Buchner, D. Hofmann, E. Surova, M. Follo, F. Kohler, H. Wardemann, K. Zirlik, H. Veelken, and H. Jumaa. 2012. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature 489:309-12.
Herzog, S., and H. Jumaa. 2012. Self-recognition and clonal selection: autoreactivity drives the generation of B cells. Curr Opin Immunol 24:166-72.
Duy, C., C. Hurtz, S. Shojaee, L. Cerchietti, H. Geng, S. Swaminathan, L. Klemm, S. M. Kweon, R. Nahar, M. Braig, E. Park, Y. M. Kim, W. K. Hofmann, S. Herzog, H. Jumaa, H. P. Koeffler, J. J. Yu, N. Heisterkamp, T. G. Graeber, H. Wu, B. H. Ye, A. Melnick, and M. Muschen. 2011. BCL6 enables Ph+ acute lymphoblastic leukaemia cells to survive BCR-ABL1 kinase inhibition. Nature 473:384-8.
Eschbach, C., M. P. Bach, I. Fidler, R. Pelanda, F. Kohler, K. Rajewsky, and H. Jumaa. 2011. Efficient generation of B lymphocytes by recognition of self-antigens. Eur J Immunol 41:2397-403.
Nahar, R., P. Ramezani-Rad, M. Mossner, C. Duy, L. Cerchietti, H. Geng, S. Dovat, H. Jumaa, B. H. Ye, A. Melnick, and M. Muschen. 2011. Pre-B cell receptor-mediated activation of BCL6 induces pre-B cell quiescence through transcriptional repression of MYC. Blood 118:4174-8.
Hurtz, C., K. Hatzi, L. Cerchietti, M. Braig, E. Park, Y. M. Kim, S. Herzog, P. Ramezani-Rad, H. Jumaa, M. C. Muller, W. K. Hofmann, A. Hochhaus, B. H. Ye, A. Agarwal, B. J. Druker, N. P. Shah, A. M. Melnick, and M. Muschen. 2011. BCL6-mediated repression of p53 is critical for leukemia stem cell survival in chronic myeloid leukemia. J Exp Med 208:2163-74.
Buchner, M., C. Baer, G. Prinz, C. Dierks, M. Burger, T. Zenz, S. Stilgenbauer, H. Jumaa, H. Veelken, and K. Zirlik. 2010. Spleen tyrosine kinase inhibition prevents chemokine- and integrin-mediated stromal protective effects in chronic lymphocytic leukemia. Blood 115:4497-506.
Werner, M., E. Hobeika, and H. Jumaa. 2010. Role of PI3K in the generation and survival of B cells. Immunol Rev 237:55-71.
Duy, C., J. J. Yu, R. Nahar, S. Swaminathan, S. M. Kweon, J. M. Polo, E. Valls, L. Klemm, S. Shojaee, L. Cerchietti, W. Schuh, H. M. Jack, C. Hurtz, P. Ramezani-Rad, S. Herzog, H. Jumaa, H. P. Koeffler, I. M. de Alboran, A. M. Melnick, B. H. Ye, and M. Muschen. 2010. BCL6 is critical for the development of a diverse primary B cell repertoire. J Exp Med 207:1209-21.
Ubelhart, R., M. P. Bach, C. Eschbach, T. Wossning, M. Reth, and H. Jumaa. 2010. N-linked glycosylation selectively regulates autonomous precursor BCR function. Nat Immunol 11:759-65.
Buchner, M., S. Fuchs, G. Prinz, D. Pfeifer, K. Bartholome, M. Burger, N. Chevalier, L. Vallat, J. Timmer, J. G. Gribben, H. Jumaa, H. Veelken, C. Dierks, and K. Zirlik. 2009. Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res 69:5424-32.
Klemm, L., C. Duy, I. Iacobucci, S. Kuchen, G. von Levetzow, N. Feldhahn, N. Henke, Z. Li, T. K. Hoffmann, Y. M. Kim, W. K. Hofmann, H. Jumaa, J. Groffen, N. Heisterkamp, G. Martinelli, M. R. Lieber, R. Casellas, and M. Muschen. 2009. The B cell mutator AID promotes B lymphoid blast crisis and drug resistance in chronic myeloid leukemia. Cancer Cell 16:232-45.
Huck, K., O. Feyen, T. Niehues, F. Ruschendorf, N. Hubner, H. J. Laws, T. Telieps, S. Knapp, H. H. Wacker, A. Meindl, H. Jumaa, and A. Borkhardt. 2009. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J Clin Invest 119:1350-8.
Herzog, S., M. Reth, and H. Jumaa. 2009. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat Rev Immunol 9:195-205.
Trageser, D., I. Iacobucci, R. Nahar, C. Duy, G. von Levetzow, L. Klemm, E. Park, W. Schuh, T. Gruber, S. Herzog, Y. M. Kim, W. K. Hofmann, A. Li, C. T. Storlazzi, H. M. Jack, J. Groffen, G. Martinelli, N. Heisterkamp, H. Jumaa, and M. Muschen. 2009. Pre-B cell receptor-mediated cell cycle arrest in Philadelphia chromosome-positive acute lymphoblastic leukemia requires IKAROS function. J Exp Med 206:1739-53.
Kohler, F., E. Hug, C. Eschbach, S. Meixlsperger, E. Hobeika, J. Kofer, H. Wardemann, and H. Jumaa. 2008. Autoreactive B cell receptors mimic autonomous pre-B cell receptor signaling and induce proliferation of early B cells. Immunity 29:912-21.
Herzog, S., E. Hug, S. Meixlsperger, J. H. Paik, R. A. DePinho, M. Reth, and H. Jumaa. 2008. SLP-65 regulates immunoglobulin light chain gene recombination through the PI(3)K-PKB-Foxo pathway. Nat Immunol 9:623-31.
Feldhahn, N., N. Henke, K. Melchior, C. Duy, B. N. Soh, F. Klein, G. von Levetzow, B. Giebel, A. Li, W. K. Hofmann, H. Jumaa, and M. Muschen. 2007. Activation-induced cytidine deaminase acts as a mutator in BCR-ABL1-transformed acute lymphoblastic leukemia cells. J Exp Med 204:1157-66.
Storch, B., S. Meixlsperger, and H. Jumaa. 2007. The Ig-alpha ITAM is required for efficient differentiation but not proliferation of pre-B cells. Eur J Immunol 37:252-60.
Thompson, E. C., B. S. Cobb, P. Sabbattini, S. Meixlsperger, V. Parelho, D. Liberg, B. Taylor, N. Dillon, K. Georgopoulos, H. Jumaa, S. T. Smale, A. G. Fisher, and M. Merkenschlager. 2007. Ikaros DNA-binding proteins as integral components of B cell developmental-stage-specific regulatory circuits. Immunity 26:335-44.
Meixlsperger, S., F. Kohler, T. Wossning, M. Reppel, M. Muschen, and H. Jumaa. 2007. Conventional light chains inhibit the autonomous signaling capacity of the B cell receptor. Immunity 26:323-33.
Liu, H., M. Schmidt-Supprian, Y. Shi, E. Hobeika, N. Barteneva, H. Jumaa, R. Pelanda, M. Reth, J. Skok, K. Rajewsky, and Y. Shi. 2007. Yin Yang 1 is a critical regulator of B-cell development. Genes Dev 21:1179-89.
Herzog, S., and H. Jumaa. 2007. The N terminus of the non-T cell activation linker (NTAL) confers inhibitory effects on pre-B cell differentiation. J Immunol 178:2336-43.
Hobeika, E., S. Thiemann, B. Storch, H. Jumaa, P. J. Nielsen, R. Pelanda, and M. Reth. 2006. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci U S A 103:13789-94.
Herzog, S., B. Storch, and H. Jumaa. 2006. Dual role of the adaptor protein SLP-65: organizer of signal transduction and tumor suppressor of pre-B cell leukemia. Immunol Res 34:143-55.
Klein, F., N. Feldhahn, S. Herzog, M. Sprangers, J. L. Mooster, H. Jumaa, and M. Muschen. 2006. BCR-ABL1 induces aberrant splicing of IKAROS and lineage infidelity in pre-B lymphoblastic leukemia cells. Oncogene 25:1118-24.
Kersseboom, R., V. B. Ta, A. J. Zijlstra, S. Middendorp, H. Jumaa, P. F. van Loo, and R. W. Hendriks. 2006. Bruton's tyrosine kinase and SLP-65 regulate pre-B cell differentiation and the induction of Ig light chain gene rearrangement. J Immunol 176:4543-52.
Sprangers, M., N. Feldhahn, S. Liedtke, H. Jumaa, R. Siebert, and M. Muschen. 2006. SLP65 deficiency results in perpetual V(D)J recombinase activity in pre-B-lymphoblastic leukemia and B-cell lymphoma cells. Oncogene 25:5180-6.
Sprangers, M., N. Feldhahn, S. Herzog, M. L. Hansmann, M. Reppel, J. Hescheler, H. Jumaa, R. Siebert, and M. Muschen. 2006. The SRC family kinase LYN redirects B cell receptor signaling in human SLP65-deficient B cell lymphoma cells. Oncogene 25:5056-62.
Wossning, T., S. Herzog, F. Kohler, S. Meixlsperger, Y. Kulathu, G. Mittler, A. Abe, U. Fuchs, A. Borkhardt, and H. Jumaa. 2006. Deregulated Syk inhibits differentiation and induces growth factor-independent proliferation of pre-B cells. J Exp Med 203:2829-40.
Feldhahn, N., F. Klein, J. L. Mooster, P. Hadweh, M. Sprangers, M. Wartenberg, M. M. Bekhite, W. K. Hofmann, S. Herzog, H. Jumaa, J. D. Rowley, and M. Muschen. 2005. Mimicry of a constitutively active pre-B cell receptor in acute lymphoblastic leukemia cells. J Exp Med 201:1837-52.
Feldhahn, N., P. Rio, B. N. Soh, S. Liedtke, M. Sprangers, F. Klein, P. Wernet, H. Jumaa, W. K. Hofmann, H. Hanenberg, J. D. Rowley, and M. Muschen. 2005. Deficiency of Bruton's tyrosine kinase in B cell precursor leukemia cells. Proc Natl Acad Sci U S A 102:13266-71.
Kohler, F., B. Storch, Y. Kulathu, S. Herzog, S. Kuppig, M. Reth, and H. Jumaa. 2005. A leucine zipper in the N terminus confers membrane association to SLP-65. Nat Immunol 6:204-10.
Middendorp, S., A. J. Zijlstra, R. Kersseboom, G. M. Dingjan, H. Jumaa, and R. W. Hendriks. 2005. Tumor suppressor function of Bruton tyrosine kinase is independent of its catalytic activity. Blood 105:259-65.
Jumaa, H., R. W. Hendriks, and M. Reth. 2005. B cell signaling and tumorigenesis. Annu Rev Immunol 23:415-45.
Parker, M. J., S. Licence, L. Erlandsson, G. R. Galler, L. Chakalova, C. S. Osborne, G. Morgan, P. Fraser, H. Jumaa, T. H. Winkler, J. Skok, and I. L. Martensson. 2005. The pre-B-cell receptor induces silencing of VpreB and lambda5 transcription. EMBO J 24:3895-905.
Nichols, K. E., K. Haines, P. S. Myung, S. Newbrough, E. Myers, H. Jumaa, D. J. Shedlock, H. Shen, and G. A. Koretzky. 2004. Macrophage activation and Fcgamma receptor-mediated signaling do not require expression of the SLP-76 and SLP-65 adaptors. J Leukoc Biol 75:541-52.
Su, Y. W., S. Herzog, M. Lotz, N. Feldhahn, M. Muschen, and H. Jumaa. 2004. The molecular requirements for LAT-mediated differentiation and the role of LAT in limiting pre-B cell expansion. Eur J Immunol 34:3614-22.
Su, Y. W., and H. Jumaa. 2003. LAT links the pre-BCR to calcium signaling. Immunity 19:295-305.
Jumaa, H., L. Bossaller, K. Portugal, B. Storch, M. Lotz, A. Flemming, M. Schrappe, V. Postila, P. Riikonen, J. Pelkonen, C. M. Niemeyer, and M. Reth. 2003. Deficiency of the adaptor SLP-65 in pre-B-cell acute lymphoblastic leukaemia. Nature 423:452-6.
Su, Y. W., A. Flemming, T. Wossning, E. Hobeika, M. Reth, and H. Jumaa. 2003. Identification of a pre-BCR lacking surrogate light chain. J Exp Med 198:1699-706.
Gerlach, J., S. Ghosh, H. Jumaa, M. Reth, J. Wienands, A. C. Chan, and L. Nitschke. 2003. B cell defects in SLP65/BLNK-deficient mice can be partially corrected by the absence of CD22, an inhibitory coreceptor for BCR signaling. Eur J Immunol 33:3418-26.
Flemming, A., T. Brummer, M. Reth, and H. Jumaa. 2003. The adaptor protein SLP-65 acts as a tumor suppressor that limits pre-B cell expansion. Nat Immunol 4:38-43.
Kersseboom, R., S. Middendorp, G. M. Dingjan, K. Dahlenborg, M. Reth, H. Jumaa, and R. W. Hendriks. 2003. Bruton's tyrosine kinase cooperates with the B cell linker protein SLP-65 as a tumor suppressor in Pre-B cells. J Exp Med 198:91-8.
Jumaa, H., M. Mitterer, M. Reth, and P. J. Nielsen. 2001. The absence of SLP65 and Btk blocks B cell development at the preB cell receptor-positive stage. Eur J Immunol 31:2164-9.
Jumaa, H., and P. J. Nielsen. 2000. Regulation of SRp20 exon 4 splicing. Biochim Biophys Acta 1494:137-43.
Jumaa, H., B. Wollscheid, M. Mitterer, J. Wienands, M. Reth, and P. J. Nielsen. 1999. Abnormal development and function of B lymphocytes in mice deficient for the signaling adaptor protein SLP-65. Immunity 11:547-54.
Jumaa, H., G. Wei, and P. J. Nielsen. 1999. Blastocyst formation is blocked in mouse embryos lacking the splicing factor SRp20. Curr Biol 9:899-902.
Wienands, J., J. Schweikert, B. Wollscheid, H. Jumaa, P. J. Nielsen, and M. Reth. 1998. SLP-65: a new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation. J Exp Med 188:791-5.
Jumaa, H., J. L. Guenet, and P. J. Nielsen. 1997. Regulated expression and RNA processing of transcripts from the Srp20 splicing factor gene during the cell cycle. Mol Cell Biol 17:3116-24.
Jumaa, H., and P. J. Nielsen. 1997. The splicing factor SRp20 modifies splicing of its own mRNA and ASF/SF2 antagonizes this regulation. EMBO J 16:5077-85.