Molecular Pathogenesis and Progression of Lymphoproliferative Disorders
Project Leader
Contact/Address
Department of Internal Medicine III
University Hospital of Ulm
Albert-Einstein-Allee 23
89081 Ulm
Germany
Phone (direct): +49-(0)731-500-45521
Phone (study office): +49-(0)731-500-45901
Fax: +49-(0)731-500-45925
Scientific Lab Members
Technicians
Christina Brücken
Jacqueline Fiegel
Elisa Graf
Bettina Hussak
Franziska Ott
Natalie Paul
Sabrina Rau
Sandra Richter
Sabrina Schrell
Julia Sempf
Research Fields
(A)
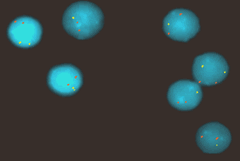
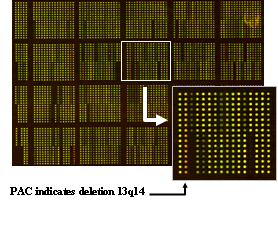
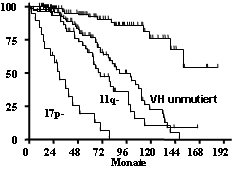
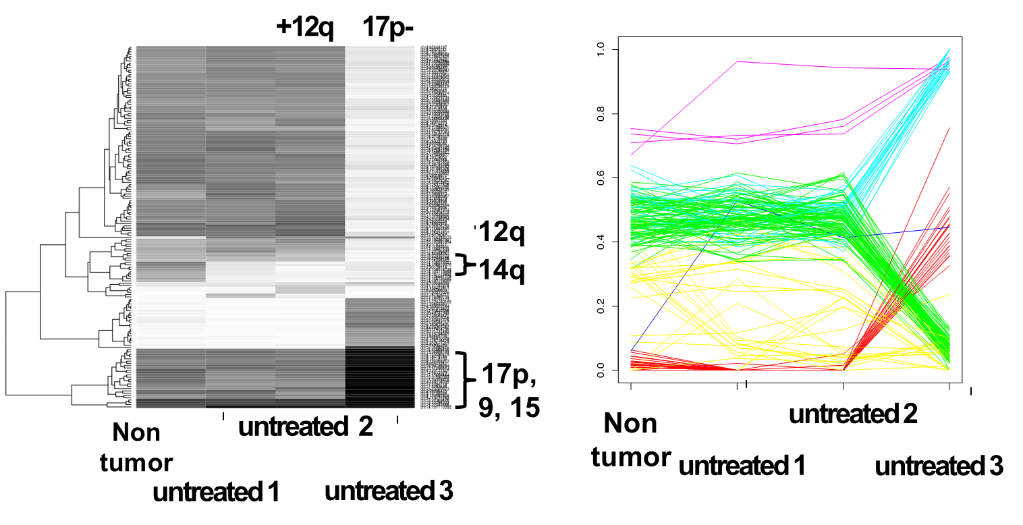
Incidence of genomic aberrations of CLL and mantle cell lymphoma (MCL). Analysis by fluorescence in-situ hybridization (FISH) with comprehensive probe sets for +(3q26), del(6q21), +(8q24), del(11q22-23), +(12q13), del(13q14), t(11;14)(q13;q32), t(14;18)(q32;q21), t(14q32), del(17p13) in CLL and in addition for del(1p22), del(6q27), +(7p15), del(8p22), del(9p21), del(10p15), +(15q23), del(18p11), +(Xq28) in MCL. Development of automated high-resolution genotyping through microarray based matrix-CGH and SNP-chip analyses.
B
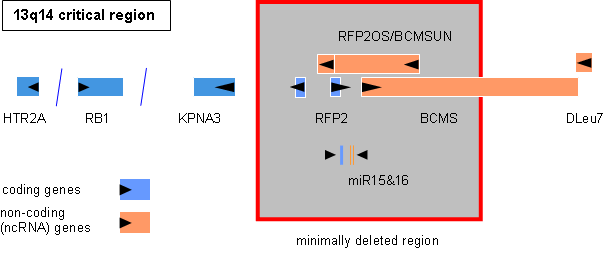
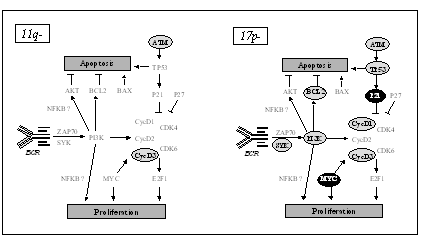
Characterization of critical regions and isolation of candidate genes involved in pathogenesis and clinical progression. For the most frequently involved genomic regions such as del(1p22), +(3q26), del(6q21), del(8p22), +(8q24), del(11q22-23), +(12q13), and del(13q14) the affected genes are mostly unknown. The critical regions are defined and candidate genes are isolated and subjected to expression as well as mutation analyses.
(C)
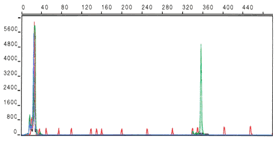
Identification of VDJ rearrangement and IGHV mutation status in lymphoproliferative disorders. Amplification of VDJ rearrangements from RNA and DNA by GeneScan technology based multiplex PCR and sequencing of the products. Characterization of VDJ structure, VH gene usage and mutation status in various B-cell malignancies to define their role in pathogenesis and clinical course of the disease.
(D)
Gene expression profiling of lymphoproliferative disorders. Deregulation of gene expression (mRNA and miR) is studied through microarray and TaqMan approaches in relation to disease subgroups defined by molecular and clinical markers. Changes in gene expression are studied during disease evolution and under specific treatments. Protein expression of candidate genes, in particular of cell cycle, apoptosis control are studied by Western blot analysis.
(F)
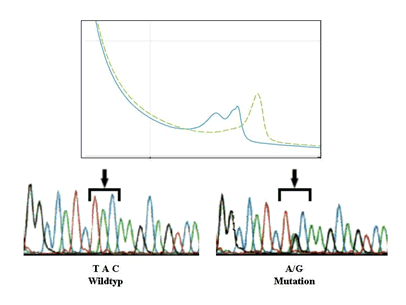
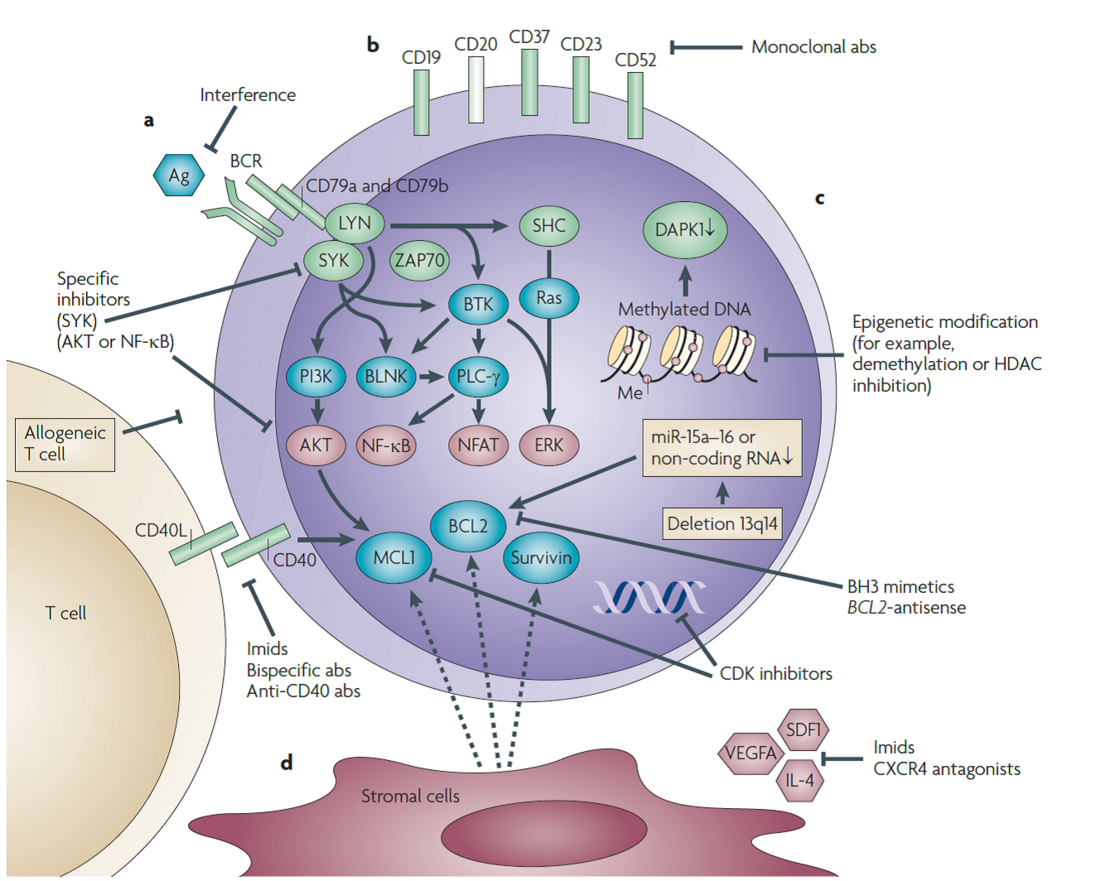
Mutation profiling of lymphoproliferative disorders. Mutations of different genes (e.g. TP53, ATM, MDM2, etc.) involved in the pathogenesis are studied by DHPLC (Denaturing High Performance Liquid Chromatography) and direct sequencing. The results are analysed in relation to disease subgroups defined by molecular and clinical markers. The consequences of gene mutations are characterised by the analysis of changes or signatures in gene expression, protein expression and functional assays after irradiation or chemotherapy.
(G)
Genomics analyses by next generation sequencing technologies (whole exome, whole genome, RNAseq, etc.) by Illumina Hiseq and targeted resequencing by Illumina MiSeq technology. Identification of pathogenic mechanisms in the development and progression of malignancies as well as in clonal evolution and treatment resistance. Subgroup definition for personalized treatment in a “precision medicine” approach.
(H)
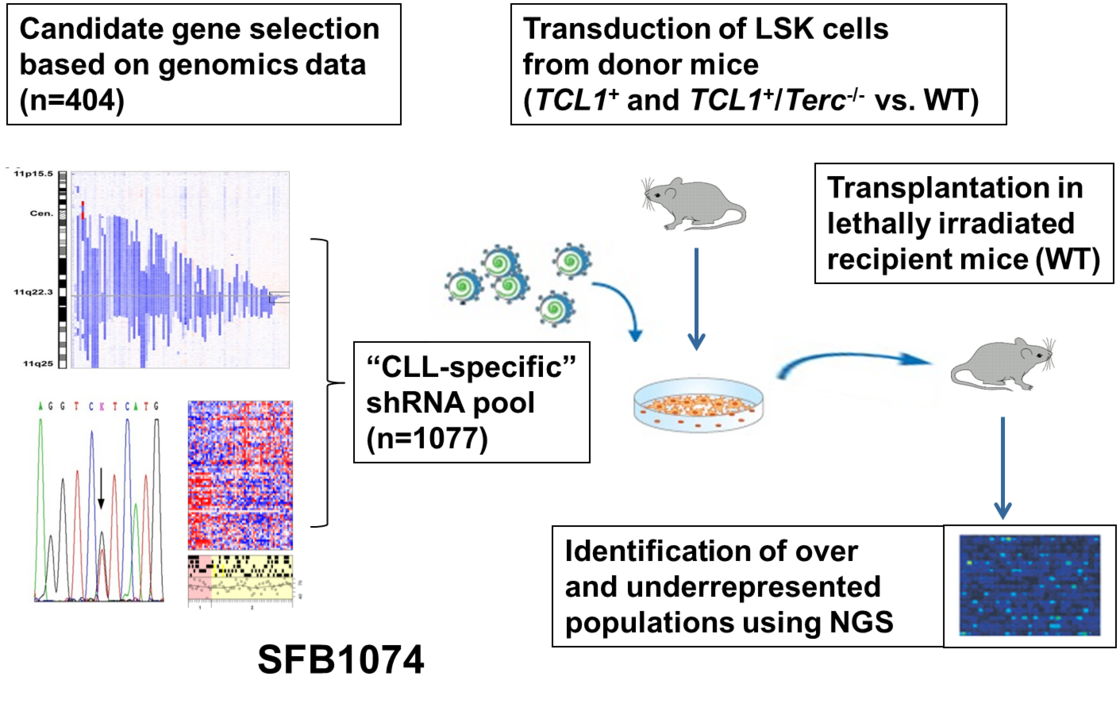
Model systems for pathogenesis and resistance mechanisms in lymphoid malignancies. Cell lines and co-culture approaches including manipulation and interference (shRNA, lentiviral infection, CRISPR technology). Mouse models involving adoptive transfer and transplantation studies as well as generation of new transgenic animals.
(I)
Central genetics reference laboratory of the German CLL Study Group (GCLLSG) focused on characterization of genomic aberrations, mutations, gene expression, cell cycle/apoptosis and tumor bank. Development of databases and compilation of datasets for comprehensive biologic and clinical characterization of leukemia and lymphoma. Identification of molecular risk markers predicting progression, treatment response, and survival. Development and coordination of clinical trial protocols based on molecular risk-assessment with innovative treatment approaches (risk stratification, resistance mechanisms, small molecules, antibody therapy, stem cell transplantation)
Techniques
- Isolation of tumor cells by ficoll gradient and immunomagnetic cell sorting
- fluorescence in-situ hybridization (FISH), also in combination with immunostaining
- DNA and RNA preparation of cells and tissues
- PCR and RT-PCR techniques, (including multiplex and TaqMan analyses)
- DNA sequencing, sequence analysis cloning and preparation of various vector systems (plasmids, cosmids, BAC, PAC, YAC)
- microarray expression (mRNA and miR) and genomic profiling (SNP chip).
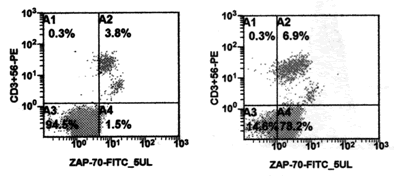
- Flow cytometry (FACS) of diverse intracellular antigens (ZAP-70 etc.)
- Sensitive mutation profiling of different genes (e.g. TP53, ATM, MDM2, etc.) by DHPLC (WAVE)
- Next generation sequencing techniques (targeted and whole exome / whole genome) by Illumina MiSeq and Illumina HiSeq technology
- Mouse models (transplantation and transgenic)
Grants/Funding
- DFG
- Deutsche Krebshilfe
- Deutsche José Carreras Leukämie-Stiftung
- Else Kröner-Fresenius Stiftung
- Wilhelm Sander Stiftung
- Land Baden-Württemberg, Bundesministerium für Bildung und Forschung,
- Europäische Kommissio
- Helmholtz Gemeinschaft
- CLL Global Research Foundation
- Industrie, etc.
Representative Publications
Representative publications can be found in the entire list of publications.
Collaboration Partners
- Prof. Dr. P. Lichter, DKFZ, Heidelberg: Molekulare Pathogenese, Progression und Resistenzmechanismen bei hämatologisch-onkologischen Erkrankungen
- Prof. Dr. C. Plass, DKFZ, Heidelberg,: Epigenomik Analysen und funktionelle Charakterisierung von Kandidatengenen
- Dipl. Stat. A. Benner, Bioinformatik, DKFZ, Heidelberg: Bioinformatische Analysen komplexer biologischer und klinischer Datensätze
- Prof. Dr. P. Möller, Pathologie, Ulm: Molekulare Pathogenese und Progression maligner hämatologischer Systemerkrankungen
- Prof. Dr. P. Gierschik, Pharmakologie, Ulm: Pathogenese und Resistenzmechanismen gegen neue „targeted drugs“ bei lymphatischen Neoplasien
- Prof. Dr. H. Jumaa, Immunologie, Ulm: Rezeptor Signaltransduktion und molekulare Mechanismen in Maus und Mensch Modellen
- Prof. Dr. S. Fulda, Frankfurt: Apoptosedefekte und deren Nutzung als therapeutische Ziele
- Prof. Dr. K.L. Rudolph, Direktor, Leibniz Institut für Alterungsforschung, Jena: Tumorstammzellen, Telomere und Seneszenz
- Prof. Dr. A. Rosenwald, Pathologie, Würzburg: Genetik, Genexpression und zelluläre Modelle bei Non-Hodgkin Lymphomen
- Prof. Dr. med. P. Dreger, Innere Medizin V, Heidelberg: Weiterentwicklung der hämatopoetischen Stammzelltransplantation
- Prof. Dr. M. Hallek, Innere Medizin I, Köln: Übertragung laborwissenschaftlicher Konzepte zur Pathogenese und Prognoseabschätzung in klinische Therapiestudien
- Prof. Dr. med J. Schetelig, Medizinische Klinik I, Dresden: Allogene Stammzelltransplantation bei der CLL (EBMT, Dresdner Studien)
- Prof. Dr. R. Rosenquist, Uppsala: Biologische und klinische Charakterisierung der CLL mit V3-21 Genbenutzung
- Prof. Dr. U. Jäger, Wien: Kokultur, Microenvironment, Signaltransduktion und Genexpression bei der CL
- Prof. Dr. F. Cymbalista, Paris: Biologie lymphatischer Neoplasien in Gewebe und Heterogenität in verschiedenen Manifestationen
- Prof. Dr. J. Gribben, London: Molekulare und zelluläre Charakterisierung von Pathogenese und Resistenz der CLL in Modellen der Maus und Mensch
- Prof. Dr. N. Chiorazzi, New-York: Pathogenetische Bedeutung des B-Zellrezeptors und der Signaltransduktion sowie Transplantationsmodelle
- Prof. Dr. R. Dalla-Favera, New-York: Molekulare und funktionelle Untersuchung lymphatischer Neoplasien in Mausmodellen
- Prof. Dr. C. Wu, Harvard Medical School und Broad Institute, Boston: Next Generation Sequencing und Tumor Heterogenität
- Prof. Dr. G. Getz, Harvard Medical School und Broad Institute, Boston: Bioinformatik und Analyse komplexer Next Generation Sequencing Datensätze
- Industriekooperationen: Abbott, Amgen, Bayer, Boehringer Ingelheim, Celgene, Gilead, Genzyme, GSK, Hoffmann-La Roche, Janssen, Mundipharma, Novartis, Sanofi-Aventis, Schering, Wyeth
- SFB1074
- Deutsche Krebshilfe "Molekulare Mechanismen bei malignen Lymphomen"
- German CLL Study Group (GCLLSG)
- European Leukemia Net (ELN)
- European Mantle Cell Lymphoma Network
- European Research Initiative on CLL (ERIC)