Research Topics
Endocrine pancreas: tailoring beta cells and characterizing genetic variants
The pancreas is a key organ exhibiting both exocrine and endocrine functions essential for food digestion and blood glucose regulation. Destruction of the endocrine pancreas by either exogenous or endogenous factors such as autoimmune reaction against β-cells, genetic predisposition or overweight causes diabetes mellitus, for which curative therapies are desperately lacking since current available treatments do not provide proper long-term glycemic control, leading to subsequent complications. Monogenic diabetes accounts for approximately 1-2% of diabetes cases and results from mutations that primarily reduce β-cell function. The identification of the genetic basis of these diabetes forms has translated into novel avenues of personalized medicine in the entire diabetes field.
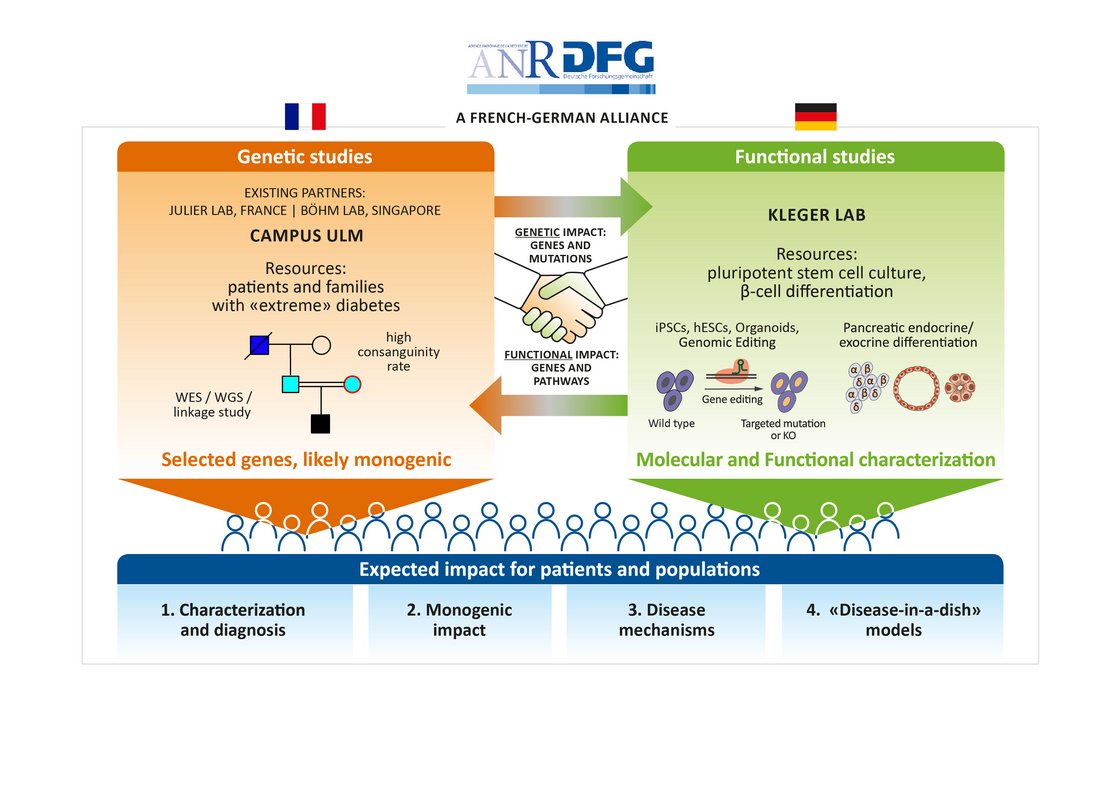
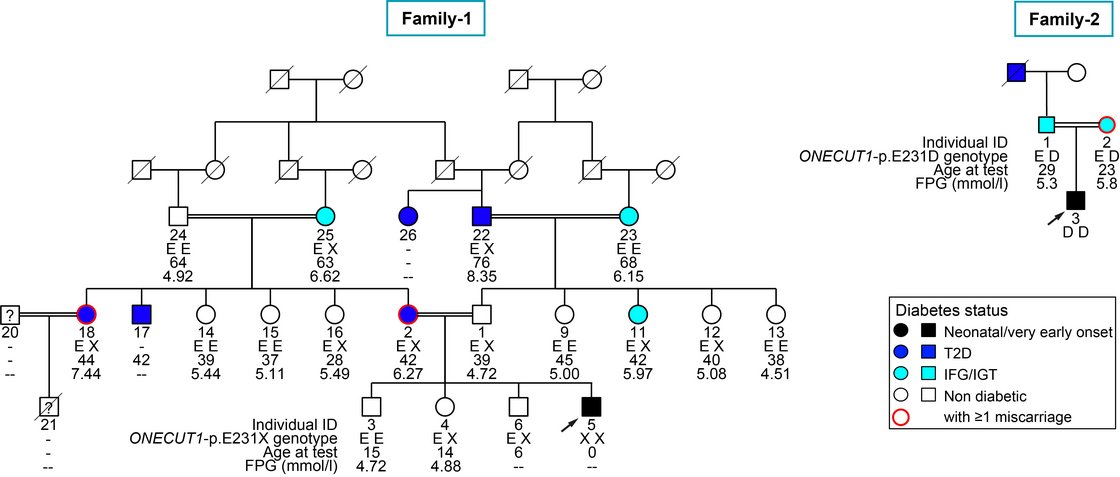
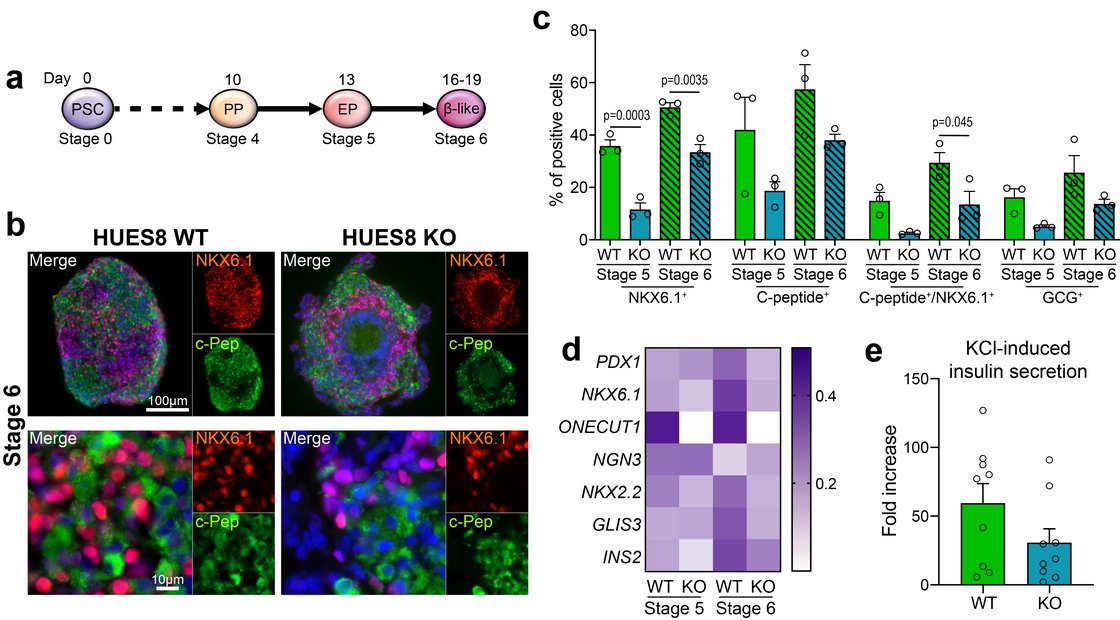
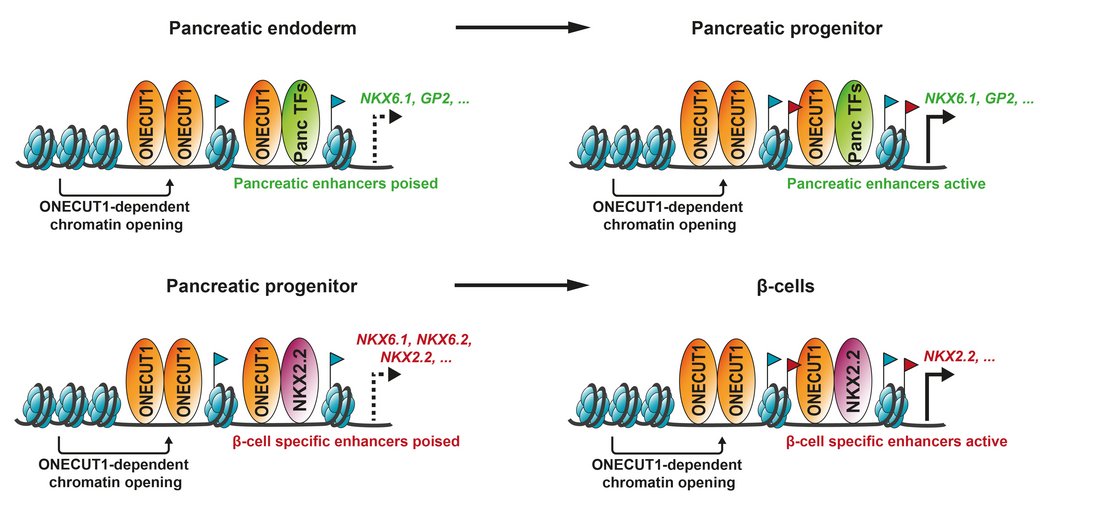
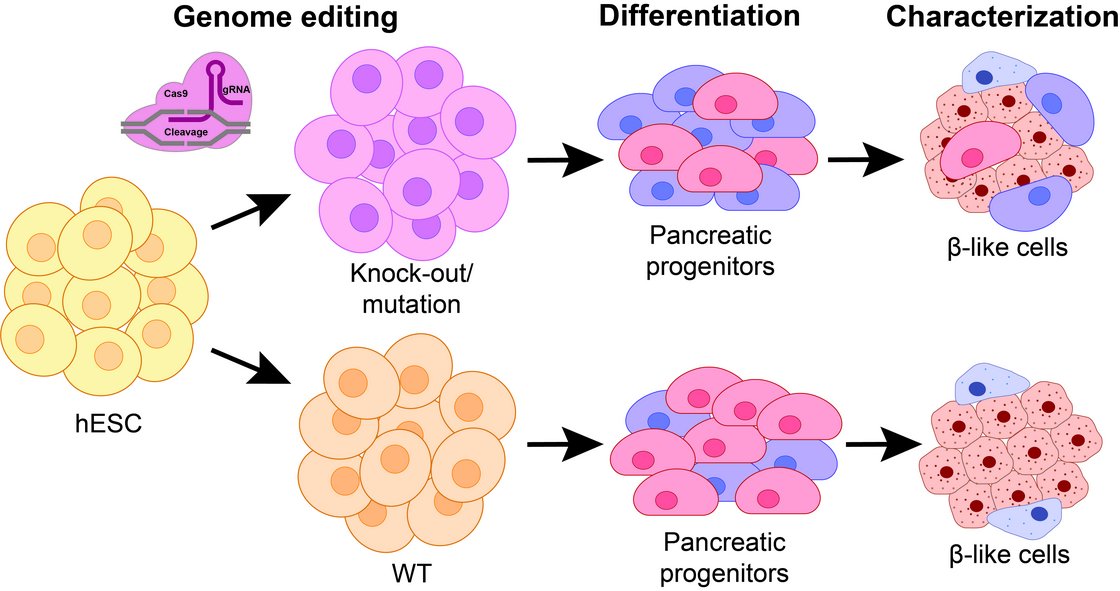
In cooperation with Prof. Cécile Julier at Université de Paris, Institut Cochin, we have identified several new genes being linked to monogenic diabetes by genome wide association studies. Our most recent work “Mutations and variants of ONECUT1 in diabetes” published in Nature Medicine (2021) and “Transcriptional changes and the role of ONECUT1 in hPSC pancreatic differentiation” (Communications Biology, 2021) describes ONECUT1 as novel diabetes gene involved in monogenic as well as multifactorial type 2 diabetes suggesting shared disease mechanisms. We used genome-edited and patient-derived pluripotent stem cells (PSC) recapitulating patient genotypes for subsequent pancreatic in vitro differentiation. These cells differentiated to functional beta-like cells and the respective intermediate stages were profiled by transcriptomics and proteomics. Results revealed that ONECUT1 is a crucial fate regulator of the transcriptional and epigenetic network during pancreatic development. Loss of ONECUT1 leads to an impaired formation of pancreatic progenitors and disturbs the following endocrine transcriptional program and thus, contributing to diabetes pathogenesis.
Our research highlights the combination of clinical studies, human genetics and in vitro differentiation of human PSCs to identify and characterize novel diabetes genes. In turn, better understanding of molecular pathomechanisms in diabetes might further improve the development of personalized therapies.
Current and future studies in our institute aim to dissect the functional role of further candidate genes identified in our genetic screening by using our established stem cell model system. Moreover, ongoing work improves currently existing pancreatic in vitro differentiation protocols to yield more pure and functional endocrine cells.
All in all, we hope to get deeper insights into the transcriptional landscape during pancreatic development as well as its deregulation in genetically caused diabetes finally using our molecular knowledge to advance personalized treatment options and quality of life for diabetes patients.
Human pancreas as a target of SARS-CoV-2 infection
The current pandemic coronavirus has spread rapidly over the world to become the major medical and socioeconomic burden. Besides infection of the respiratory tract, other organs such as the pancreas can be affected. While preexisting diabetes can aggravate coronavirus disease 2019 (COVID-19), cases of new onset or exacerbated diabetes have been reported. In order to better understand the multi-organ manifestation and the underlying pathomechanisms, suitable model systems are essential.
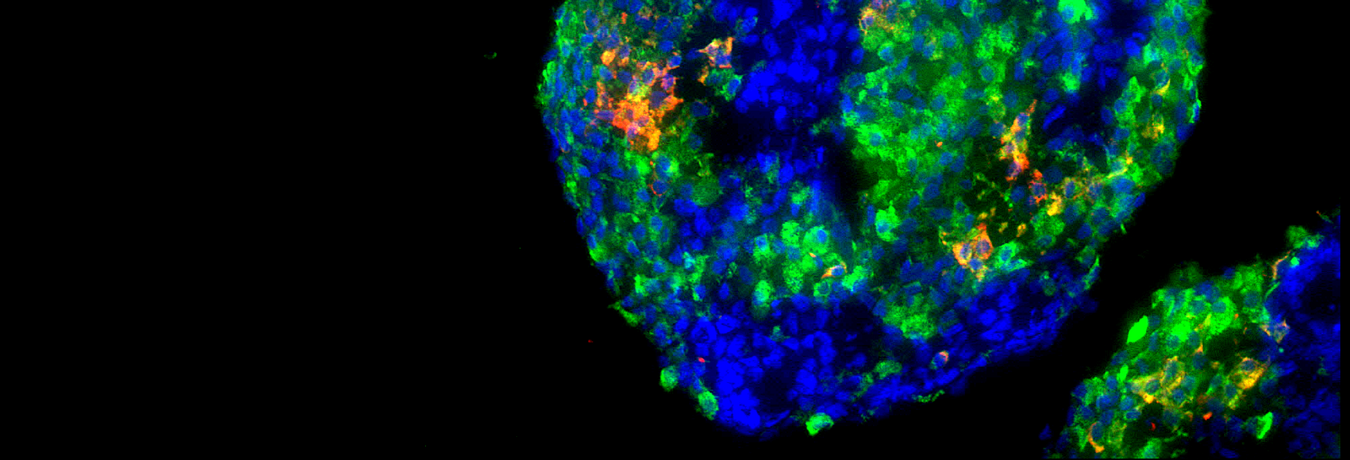
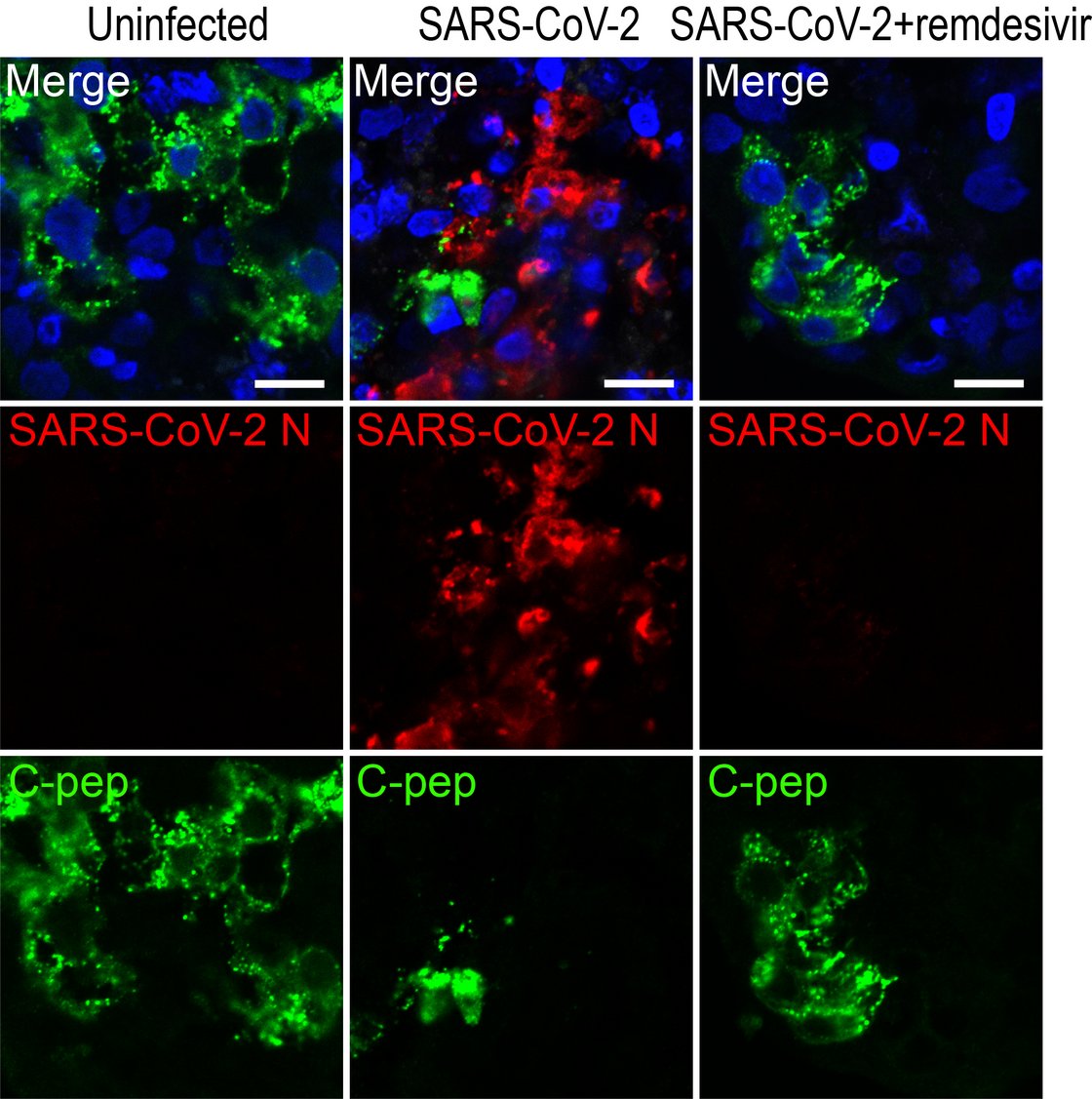
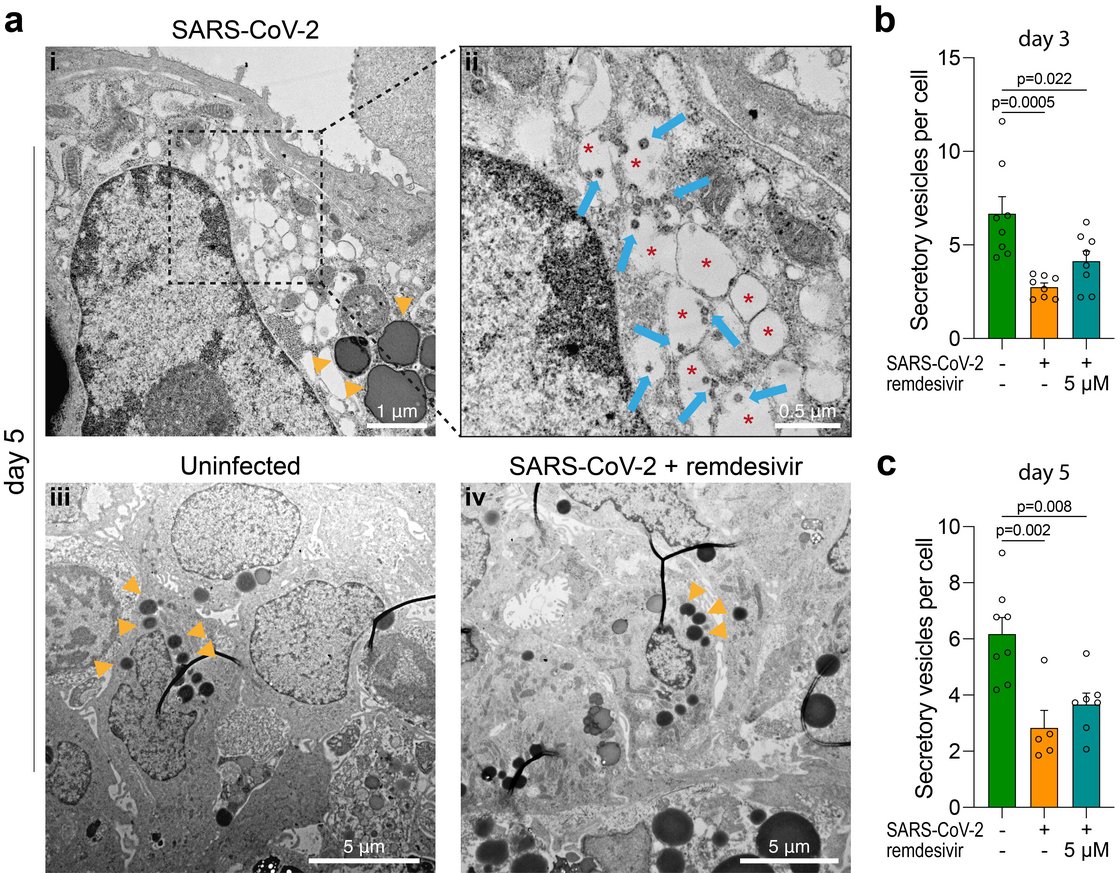
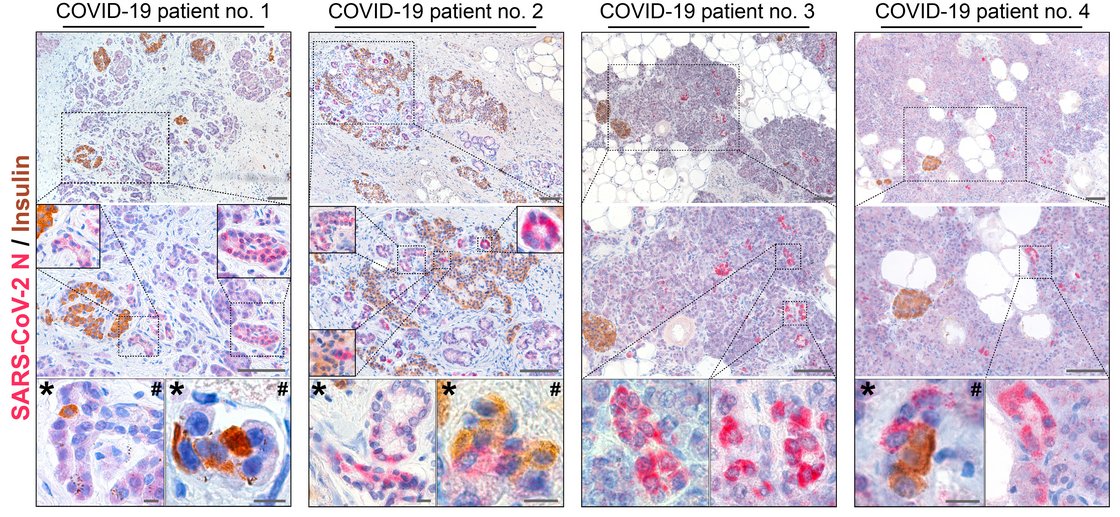
Insight into these processes revealed our recent work “SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas” published in Nature Metabolism (2021). This collaborative study with Prof. Jan Münch at the Institute of Molecular Virology (Ulm University Medical Center) showed that SARS-CoV-2 infects human pancreatic exocrine and endocrine cells. Moreover, we demonstrate viral infection and replication in cultured human islets which is accompanied by morphological, transcriptional and functional changes. This suggests that pancreatic infection could contribute to endocrine dysfunction observed in COVID-19 patients, however, more detailed studies are needed to decipher exact molecular pathways and biological processes.
As a possible model system, human islets of Langerhans preparations not suitable for transplantation have limited availability, are very expensive and often have low preparation quality and purity. Moreover, donor diversity and restricted material requires multipleislet preparations to generate robust results. Therefore, we will apply stem cell-derived pancreatic beta-like cells to dissect the consequences of pancreatic SARS-CoV-2 infection on beta-cell function and the transcriptional network. This will help to better understand the immediate and long-term effects within the pancreatic endocrine compartment and to develop potential medical interventions.