(Neuro)Inflammation
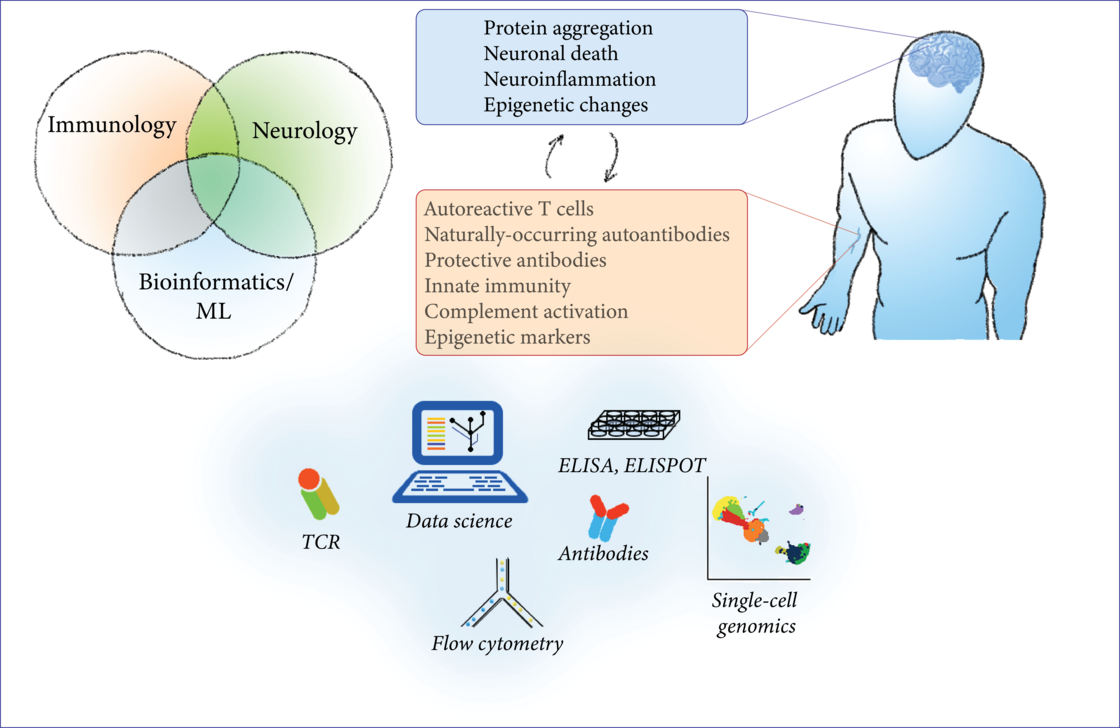
The immune system offers several unique chances for the research of neurodegenerative diseases: biomarker research, investigation of disease mechanisms, potential therapeutic targets, and translational insights. In this project, we study the interface between the immune system and neurodegenerative diseases by a combination of modern bioinformatic and machine-learning techniques with classical molecular biology techniques from the fields of immunology and neuroscience. As an easily accessible, highly-reactive ‘mirror’ of systemic processes, the immune system reflects changes in the CNS that can be measured in peripheral blood. We characterize the composition of blood cells, their gene expression, epigenetic changes and functional aberrations in PD and ALS with flow cytometry, primary cell culture and multi-omic approaches in bulk and single-cell genomics (ATAC-seq, RNA-seq, protein expression). To search for novel disease mechanisms and potential therapeutic targets, we characterize the circulating T cells and their T-cell receptor with flow cytometry, ELISPOT, T-cell receptor sequencing and machine learning; we test the effect of recombinant antibodies to CNS antigens on protein aggregation with in-vitro assays; finally, we investigate the receptors and cytokines involved in innate immunity to aggregated proteins with ELISA and molecular biology techniques.
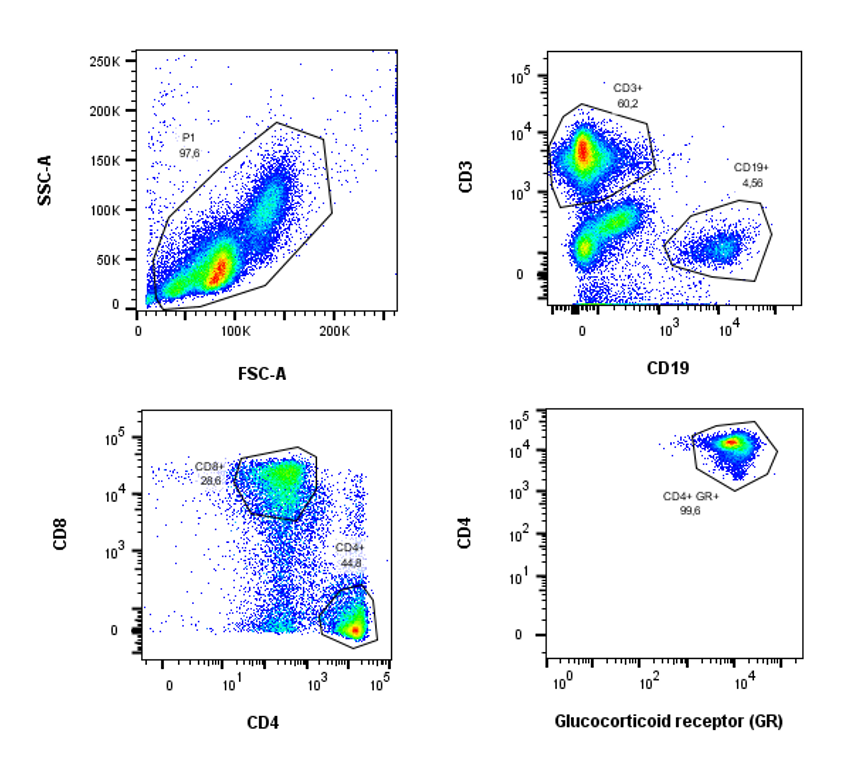
The hypothalamic-pituitary-adrenal axis (HPA) axis is one of the two major stress systems, that is activated in response to stress. Recent studies suggest that α-syn aggregation may impact the functioning of the HPA axis. Reduced ACTH levels have been observed in PD patients and altered levels of cortisol in PD patients have been reported.
In this project we aim to study the impact of α-syn on the stress-associated axis of the immune system and the glucocorticoid (GC) sensitivity of immune cells. This project is performed in collaboration with the group of Stefan Reber, who has a strong expertise in stress related changes in the immune system. Towards this end we characterize stress parameters in our PD a-syn model as well as in human peripheral blood mononuclear cells (PBMCs) of PD patients.
Autoantibodies are antibodies produced by the immune system that mistakenly target and attack the body's own tissues or proteins. Recently, autoantibodies have been identified in some cases of patients with neurodegenerative diseases, which potentially contribute to the progression or exacerbation of neurodegenerative disorders. However, the role of autoantibodies in neurodegenerative diseases and the understanding of their involvement is not clear to date.
We aim to look for circulating auto-antibodies to known and putative CNS antigens and investigate the complement activation. It is already known that the proteins TDP-43 and SOD1 are directly related to ALS disease. Especially the pathologically modified forms in the sense of aggregation and changes in localization play a role.
Therefore, in this project we investigate detection, quantitative determination, and comparative presentation of autoantibodies against ALS disease associated proteins in the human serum of ALS patients and healthy control subjects. We also want to study whether extracellular aggregate formation is possible and to what extent this process can be inhibited by the addition of antibodies.
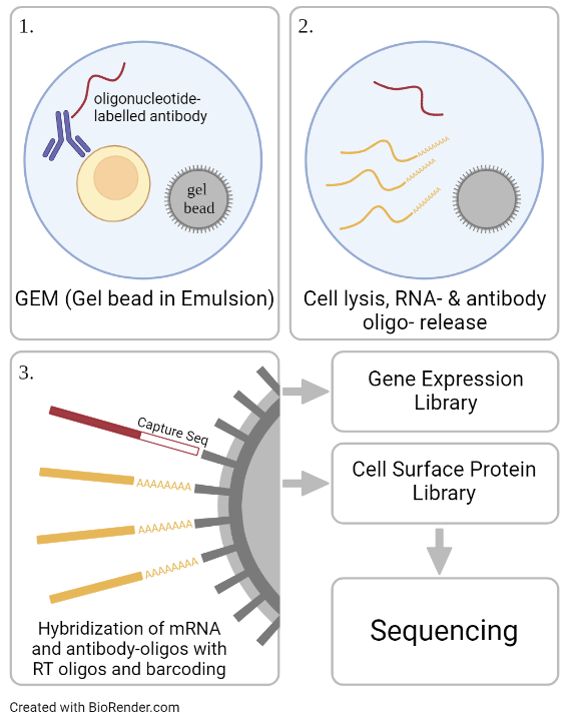
In a subset of patients, MDD is associated with inflammatory processes. To further investigate this relation, we aim to immuno-characterize peripheral blood mononuclear cells (PBMCs) from MDD patients and healthy controls using cellular indexing of transcriptomes and epitopes (CITE-Seq). Thereby, differentially expressed genes as well as changes in the cell surface proteome can be investigated on a single cell level. Additionally, extracellular vesicles are extracted from the patient’s plasma and their cargo is analyzed using mass spectrometry for further immune phenotyping as well as biomarker discovery. Structural and activity related changes have been detected in distinct brain regions of MDD patients, including the amygdala and the anterior cingulate cortex, areas that are important for cognitive and affective functions. In a multi-omic approach, chromatin accessibility and transcriptomics are analyzed on a single cell level in post mortem brain samples of MDD patients.
People

Dr. Verena Bopp
M. Sc. Molecular and Translational Neuroscience
Phone: +49731-500-63039
Fax: +49 731-50063050
Mail: verena.bopp@uni-ulm.de

Dr. Veselin Grozdanov
M.Sc. Biochemistry
Phone: +49-731-500-63152
Fax: +49-731-500-63050
Mail: veselin.grozdanov@uni-ulm.de

Laura Meier
M.Sc. Biochemistry
Phone: +49731-500-63039
Fax: +49 731-50063050
Mail: laura.meier@uni-ulm.de
